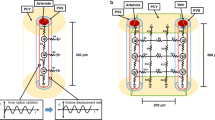
Abstract
Circulation of fluid through the central nervous system maintains fluid homeostasis and is involved in solute clearance. The glymphatic system is hypothesised to facilitate waste clearance in the brain, with inflow via periarterial spaces, bulk flow through the parenchyma, and outflow via perivenous spaces. The driving force for this mechanism is unknown. Previous modelling in the spinal cord suggests that timing offsets between arterial and subarachnoid space pressure pulses can enable net inflow in perivascular spaces (PVS). This study adapted the spinal pulse offset mechanism to the brain and simulated movement of tracer particles used in experiments. Both bulk flow and diffusive movement of tracer were simulated. Intracranial pressure pulses were applied to one end of a 300-μm-long perivascular space combined with a moving arterial wall simulating arterial pulsations. The simulations indicate the pulse offset mechanism can enable net inflow via PVS; however, it is unknown whether the temporal offset required is physiologically realistic. Increasing the positive component of the ICP (intracranial pressure) pulse increased net flow. Tracer particles driven by bulk flow reached the outlet of the PVS with a net speed of ~ 16 μm/s when the permeability was two orders of magnitude higher than values in the literature. These particles were unable to penetrate into the parenchyma in the absence of diffusion. Dispersion dominated tracer movement in the parenchyma. Further research is required to reconcile discrepancies between these results, and both experimental and computational studies.









Similar content being viewed by others
References
Asgari M, de Zelicourt D, Kurtcuoglu V (2015) How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep 5:15024
Asgari M, de Zelicourt D, Kurtcuoglu V (2016) Glymphatic solute transport does not require bulk flow. Sci Rep 6:38635
Basser PJ (1992) Interstitial pressure, volume, and flow during infusion into brain tissue. Microvasc Res 44:143–165
Bedussi B, Almasian M, de Vos J, VanBavel E, Bakker EN (2018) Paravascular spaces at the brain surface: low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab 38:719–726
Bilston LE, Stoodley MA, Fletcher DF (2010) The influence of the relative timing of arterial and subarachnoid space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx development. J Neurosurg 112:808–813
Bloomfield IG, Johnston IH, Bilston LE (1998) Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr Neurosurg 28:246–251
Czosnyka M, Czosnyka Z, Keong N, Lavinio A, Smielewski P, Momjian S, Schmidt EA, Petrella G, Owler B, Pickard JD (2007) Pulse pressure waveform in hydrocephalus: what it is and what it isn’t. Neurosurg Focus 22:E2
Daversin-Catty C, Vinje V, Mardal K-A, Rognes ME (2020) The mechanisms behind perivascular fluid flow. PLoS ONE 15:e0244442
Delaidelli A, Moiraghi A (2017) Respiration: a new mechanism for CSF circulation? J Neurosci 37:7076–7078
Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J (2015) Inspiration is the major regulator of human CSF flow. J Neurosci 35:2485–2491
Dreha-Kulaczewski S, Konopka M, Joseph AA, Kollmeier J, Merboldt KD, Ludwig HC, Gartner J, Frahm J (2018) Respiration and the watershed of spinal CSF flow in humans. Sci Rep 8:5594
Hannocks MJ, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, Sorokin L (2018) Molecular characterization of perivascular drainage pathways in the murine brain. J Cereb Blood Flow Metab 38:669–686
Hladky SB, Barrand MA (2018) Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 15:30
Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal KA, Pettersen KH (2017) Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A 114:9894–9899
Hydon PE, Pedley TJ (1993) Axial dispersion in a channel with oscillating walls 249:535–555
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes including amyloid beta. Sci Transl Med 4:147ra111
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:18190–18199
Jin BJ, Smith AJ, Verkman AS (2016) Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148:489–501
Kasprowicz M, Lalou DA, Czosnyka M, Garnett M, Czosnyka Z (2016) Intracranial pressure, its components and cerebrospinal fluid pressure-volume compensation. Acta Neurol Scand 134:168–180
Kedarasetti RT, Drew PJ, Costanzo F (2020a) Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci Rep 10:10102
Kedarasetti RT, Turner KL, Echagarruga C, Gluckman BJ, Drew PJ, Costanzo F (2020b) Functional hyperemia drives fluid exchange in the paravascular space. Fluids Barriers CNS 17:52
Keith Sharp M, Carare RO, Martin BA (2019) Dispersion in porous media in oscillatory flow between flat plates: applications to intrathecal, periarterial and paraarterial solute transport in the central nervous system. Fluids Barriers CNS 16:13
Lam MA, Hemley SJ, Najafi E, Vella NGF, Bilston LE, Stoodley MA (2017) The ultrastructure of spinal cord perivascular spaces: Implications for the circulation of cerebrospinal fluid. Sci Rep 7:12924
Lei Y, Han H, Yuan F, Javeed A, Zhao Y (2017) The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Prog Neurobiol 157:230–246
Linninger AA, Xenos M, Sweetman B, Ponkshe S, Guo X, Penn R (2009) A mathematical model of blood, cerebrospinal fluid and brain dynamics. J Math Biol 59:729–759
Lloyd RA, Fletcher DF, Clarke EC, Bilston LE (2017) Chiari malformation may increase perivascular cerebrospinal fluid flow into the spinal cord: a subject-specific computational modelling study. J Biomech 65:185–193
Martinac AD, Bilston LE (2020) Computational modelling of fluid and solute transport in the brain. Biomech Model Mechanobiol 19:781–800
Mastorakos P, McGavern D (2019) The anatomy and immunology of vasculature in the central nervous system. Sci Immunol 4
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9:4878
Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ (2002) Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (lond) 103:371–377
Mobed-Miremadi M, Djomehri S, Keralapura M, McNeil M (2014) Fickian-based empirical approach for diffusivity determination in hollow alginate-based microfibers using 2D fluorescence microscopy and comparison with theoretical predictions. Materials (basel) 7:7670–7688
Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP (1998) Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci 18:2506–2519
Numata S, Itatani K, Kawajiri H, Yamazaki S, Kanda K, Yaku H (2017) Computational fluid dynamics simulation of the right subclavian artery cannulation. J Thorac Cardiovasc Surg 154:480–487
Penn RD, Linninger A (2009) The physics of hydrocephalus. Pediatr Neurosurg 45:161–174
Rey J, Sarntinoranont M (2018) Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS 15:20
Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS (2017) Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6
Spijkerman JM, Geurts LJ, Siero JCW, Hendrikse J, Luijten PR, Zwanenburg JJM (2019) Phase contrast MRI measurements of net cerebrospinal fluid flow through the cerebral aqueduct are confounded by respiration. J Magn Reson Imaging 49:433–444
Stephensen H, Tisell M, Wikkelsö C (2002) There is no transmantle pressure gradient in communicating or noncommunicating hydrocephalus. Neurosurgery 50:763–773
Thomas JH (2019) Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface 16:20190572
Tithof J, Kelley DH, Mestre H, Nedergaard M, Thomas JH (2019) Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 16:19
Toger J, Zahr MJ, Aristokleous N, Markenroth Bloch K, Carlsson M, Persson PO (2020) Blood flow imaging by optimal matching of computational fluid dynamics to 4D-flow data. Magn Reson Med 84:2231–2245
Valnes LM, Mitusch SK, Ringstad G, Eide PK, Funke SW, Mardal K-AJ (2018) Can diffusion alone explain brain-wide distribution of a CSF tracer within 24 hours?
Vinje V, Ringstad G, Lindstrom EK, Valnes LM, Rognes ME, Eide PK, Mardal KA (2019) Respiratory influence on cerebrospinal fluid flow—a computational study based on long-term intracranial pressure measurements. Sci Rep 9:9732
Wagshul ME, Eide PK, Madsen JR (2011) The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8:5
Wang P, Olbricht WL (2011) Fluid mechanics in the perivascular space. J Theor Biol 274:52–57
Yang B, Verkman AS (1997) Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem 272:16140–16146
Funding
ADM is supported by an Australian Government Research Training Program Scholarship. LEB is supported by an NHMRC Investigator fellowship.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by ADM and LEB. The model was designed and implemented by ADM in consultation with DFF and LEB. ADM performed the simulations and drafted the manuscript with assistance from LEB and DFF. All authors interpreted the results and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource3 Video of the case shown in Figure 4A. PVS permeability was set to 2×10–14 m2, the ‘baseline’ value from previous modelling studies. The maximum penetration of the tracer particles by bulk flow was 42 μm, the particles became indefinitely trapped in PVS recirculation zones due to the restrictive permeability. The average velocity of the particles was 170 μm/s. (WMV 1484 kb)
Online Resource4 Video of the case shown in Figure 4C. PVS permeability was set to 2×10–12 m2. The maximum particle penetration was 300 μm (the entire length of the PVS) over 24 pulses. While the average instantaneous velocity of the particles was 262 μm/s, the net speed of particles was 16 μm/s. (WMV 1466 kb)
Online Resource5 Video of the case shown in Figure 9 (orange line). With a greatly reduced arterial wall displacement (1 μm) and a pressure increase of 2 Pa for the pressure curve, the tracer was able to traverse the length of the PVS (300 μm). (WMV 1259 kb)
Online Resource6 Video of the case shown in Online Resource 1, Fig OR1_8. With a greatly reduced arterial wall displacement (1 μm) and a pressure increase of 2 Pa for the pressure curve, the tracer was able to traverse the length of the PVS (300 μm). (WMV 2231 kb)
Online Resource7 Video of Online Resource 1, Fig OR1_6A Resistive block permeability of 7 × 10–13 m2. The particle pathlines are very similar to Figure 4C and it takes 41 pulses for the discrete particles to reach the PVS-Rblock boundary (similar to a net pressure of +0.5 Pa). (WMV 2244 kb)
Online Resource8 Video of Online Resource 1, Fig OR1_7A. 100 cardiac cycles with an arterial wall amplitude of 10 µm, no net pressure, and an offset of 30% between the pressure and arterial wall pulses. The particles penetrate to approximately the midpoint of the PVS and bifurcation of the particles is clearly visible. The particle pathlines are almost identical to those shown in Figure 4B. (WMV 4278 kb)
Appendices
Appendix 1
There is some controversy in the literature as to whether there is a meaningful mean pressure difference between the ventricles and SAS or between the SAS and parenchyma. However, as fluid flows from one to the other at various points in the cardiac cycle, there is clearly a dynamic pressure difference during the cardiac cycle.
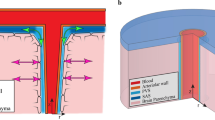
As stated in the main text in Sect. 1 ‘The impetus for investigating the pulse offset mechanism was that both the PVS associated with the artery and PVS associated with the venule might be connected to the cranial SAS’ (Hannocks et al. 2018; Mastorakos and McGavern 2019). Therefore, it seems unlikely that the pressures where they join the SAS would differ significantly. Figure
Schematic of an artery-vein pair with a parenchymal section between them. Both these vessels are connected to approximately the same region of the SAS. If this assumption is correct, then there would be no significant pressure difference between the periarterial and perivenous spaces. Blue arrows indicate possible flow directions
10 illustrates this point.
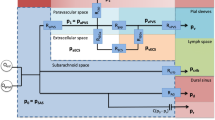
It is due to both above-mentioned factors that the pulse offset mechanism was developed under a zero-net pressure condition where the modelled pressure is the difference between the SAS and parenchyma. To create this pressure input for the model, data from Kasprowicz et al. (2016) (Fig.
Pressure and displacement boundary conditions. a Averaged ICP trace from b (triangles) converted from mmHg to Pa for use in Ansys CFX. Fourier series fit of the data (line). b Original SAS ICP pressure recording modified for the results in a and c (Kasprowicz et al. 2016). (c) Pressure pulse curve applied to the SAS (magnitude of P = 133 Pa = 1 mmHg). Area is the same above and below the curve resulting in no net pressure applied to the system over the course of 1 cycle
11). This recording was used as it showed three distinct pulses over a time scale of seconds, in humans. While the pressure recordings are slightly noisy, this was softened by taking the average of the three traces. This was done primarily to obtain a shape for the SAS ICP based on human in vivo results. The averaged result is shown in Fig. 11b along with a Fourier series fit used to further reduce the noise.
The mean of the SAS pressure from Kasprowicz et al. is ~ 27 mmHg and the initial assumption of our model is that there is no net pressure in the modelled region. Therefore, the curve in Fig. 11a is shifted to a mean of zero; the PVS outlet is also set to 0 mmHg (effectively 27 mmHg). Thus, the curve in Fig. 2b was shifted to have a mean of 0 mmHg. This ensured that the cyclic pressure added no net pressure to the system per cycle, the area above and below the curve summed to zero. The pressure was scaled to 1 mmHg based on the findings of Stephensen et al. (2002) and Penn and Linninger (2009), which show that the pressure difference between the SAS and venules, parenchyma and venules do not exceed ~ 1 mmHg leading to a further assumption that the magnitude of the pressure difference between the SAS and parenchyma would also not exceed this value. It must be stated that the results of Stephensen et al. are from humans and Penn et al. are from dog experiments.
Appendix 2
2.1 Permeability calculation
Permeability of the astrocyte end-foot layer was calculated for a single end-foot using Darcy’s law in the absence of gravitational forces. This was further multiplied by the number of end-feet in the simulated area:
where
\({\rm{Volumetric flow rate of a single AQP}}4 = 35 \times 10^{{ - 14}} {\rm{cm}}^{3} {\rm{s}}^{{ - 1}}\, \) (Yang and Verkman 1997) \(= 3.5 \times 10^{{ - 19}}\, {\rm{m}}^{3} {\rm{s}}^{{ - 1}}\)
No. of AQP4 channels per end-foot = 78 000 (Nagelhus et al. 1998) \({\rm{giving Q}} = 3.5 \times 10^{{ - 19}} \, {\rm{m}}^{3} {\rm{s}}^{{ - 1}} \times 78000 = 1.53 \times 10^{{ - 12}} {\rm{m}}^{3} {\rm{s}}^{{ - 1}}\)
\(A = {\rm{area \, of \, an \, end \,foot}} = 78\,{\upmu} {\rm{m}}^{2}\) (Asgari et al. 2015) \(= 7.8 \times 10^{{ - 11}}\, {\rm{m}}^{2}\)
\({{\Delta }}P = 1\,{\rm{mmHg}} = 133.322\,{\rm{Pa}}\)(Penn and Linninger 2009; Stephensen et al. 2002)
L = depth of the end-feet layer \(= 1{\rm{~}} \times {\rm{~}}10^{{ - 6}} {\rm{~m~}}\) (Mathiisen et al. 2010).
The area of the astrocyte layer is 300 µm (length) × 8.72 µm (width) = 2616 µm2 and the area of an end-foot is 78 µm2.
\(\therefore\) 2616/78 = 34 is the number of end-feet modelled in the astrocyte region.
Rights and permissions
About this article
Cite this article
Martinac, A.D., Fletcher, D.F. & Bilston, L.E. Phase offset between arterial pulsations and subarachnoid space pressure fluctuations are unlikely to drive periarterial cerebrospinal fluid flow. Biomech Model Mechanobiol 20, 1751–1766 (2021). https://doi.org/10.1007/s10237-021-01474-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01474-0