Abstract
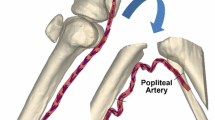
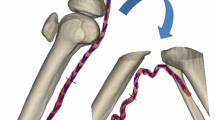
Planar biaxial testing is commonly used to characterize the mechanical properties of arteries, but stresses associated with specimen flattening during this test are unknown. We quantified flattening effects in human femoropopliteal arteries (FPAs) of different ages and determined how they affect the calculated arterial physiologic stress–stretch state. Human FPAs from 472 tissue donors (age 12–82 years, mean 53 ± 16 years) were tested using planar biaxial extension, and morphometric and mechanical characteristics were used to assess the flattening effects. Constitutive parameters for the invariant-based model were adjusted to account for specimen flattening and used to calculate the physiologic stresses, stretches, axial force, circumferential stiffness, and stored energy for the FPAs in seven age groups. Flattened specimens were overall 12 ± 4% stiffer longitudinally and 19 ± 11% stiffer circumferentially when biaxially tested. Differences between the stress–stretch curves adjusted and non-adjusted for the effects of flattening were relatively constant across all age groups longitudinally, but increased with age circumferentially. In all age groups, these differences were smaller than the intersubject variability. Physiologic stresses, stretches, axial force, circumferential stiffness, and stored energy were all qualitatively and quantitatively similar when calculated with and without the flattening effects. Stresses, stretches, axial force, and stored energy reduced with age, but circumferential stiffness remained relatively constant between 25 and 65 years of age suggesting a homeostatic target of 0.75 ± 0.02 MPa. Flattening effects associated with planar biaxial testing are smaller than the intersubject variability and have little influence on the calculated physiologic stress–stretch state of human FPAs.







Similar content being viewed by others
References
Adam DJ, Beard JD, Cleveland T et al (2005) Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 366:1925–1934. https://doi.org/10.1016/S0140-6736(05)67704-5
Alford PW, Humphrey JD, Taber LA (2008) Growth and remodeling in a thick-walled artery model: effects of spatial variations in wall constituents. Biomech Model Mechanobiol 7:245–262. https://doi.org/10.1007/s10237-007-0101-2
Brossollet LJ, Vito RP (1995) An alternate formulation of blood vessel mechanics and the meaning of the in vivo property. J Biomech 28:679–687. https://doi.org/10.1016/0021-9290(94)00119-O
Bustamante R, Holzapfel GA (2010) Methods to compute 3D residual stress distributions in hyperelastic tubes with application to arterial walls. Int J Eng Sci 48:1066–1082. https://doi.org/10.1016/j.ijengsci.2010.06.005
Cardamone L, Valentín A, Eberth JF, Humphrey JD (2009) Origin of axial prestretch and residual stress in arteries. Biomech Model Mechanobiol 8(6):431–446
Conte MS, Bandyk DF, Clowes AW et al (2006) Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 43:742–751. https://doi.org/10.1016/j.jvs.2005.12.058
Desyatova A, MacTaggart J, Kamenskiy A (2017a) Constitutive modeling of human femoropopliteal artery biaxial stiffening due to aging and diabetes. Acta Biomater 64:50–58. https://doi.org/10.1016/j.actbio.2017.09.042
Desyatova A, Poulson W, Deegan P et al (2017b) Limb flexion-induced twist and associated intramural stresses In the human femoropopliteal artery. J R Soc Interface. https://doi.org/10.1098/rsif.2017.0025
Desyatova A, Poulson W, MacTaggart J et al (2018) Cross-sectional pinching in human femoropopliteal arteries due to limb flexion, and stent design optimization for maximum cross-sectional opening and minimum intramural stresses. J R Soc Interface 15:10–14. https://doi.org/10.1098/rsif.2018.0475
Feldman SA, Glagov S (1971) Transmedial collagen and elastin gradients in human aortas: reversal with age. Atherosclerosis 13:385–394
Ferruzzi J, Vorp DA, Humphrey JD (2011) On constitutive descriptors of the biaxial mechanical behaviour of human abdominal aorta and aneurysms. J R Soc Interface 8:435–450
Ferruzzi J, Di Achille P, Tellides G, Humphrey JD (2018) Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PLoS ONE 13:1–21. https://doi.org/10.1371/journal.pone.0201379
Holzapfel GA, Ogden RW (2009) On planar biaxial tests for anisotropic nonlinearly elastic solids. A continuum mechanical framework. Math Mech Solids 14:474–489. https://doi.org/10.1177/1081286507084411
Holzapfel GA, Ogden RW (2010a) Constitutive modelling of arteries. Proc R Soc A Math Phys Eng Sci 466:1551–1597. https://doi.org/10.1098/rspa.2010.0058
Holzapfel GA, Ogden RW (2010b) Modelling the layer-specific three-dimensional residual stresses in arteries, with an application to the human aorta. J R Soc Interface 7:787–799
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Holzapfel GA, Sommer G, Auer M et al (2007) Layer-specific 3D residual deformations of human aortas with non-atherosclerotic intimal thickening. Ann Biomed Eng 35:530–545
Humphrey JD (2002) Cardiovascular solid mechanics. Springer, New York
Humphrey JD (2008) Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50:53–78. https://doi.org/10.1007/s12013-007-9002-3
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15:802–812. https://doi.org/10.1038/nrm3896
Kamenskiy A, Pipinos I, Dzenis Y et al (2014) Passive biaxial mechanical properties and in vivo axial pre-stretch of the diseased human femoropopliteal and tibial arteries. Acta Biomater 10:1301–1313. https://doi.org/10.1016/j.actbio.2013.12.027
Kamenskiy AV, Pipinos II, Dzenis YA et al (2015) Effects of age on the physiological and mechanical characteristics of human femoropopliteal arteries. Acta Biomater 11:304–313. https://doi.org/10.1016/j.actbio.2014.09.050
Kamenskiy A, Seas A, Bowen G et al (2016) In situ longitudinal pre-stretch in the human femoropopliteal artery. Acta Biomater 32:231–237. https://doi.org/10.1016/j.actbio.2016.01.002
Kamenskiy A, Seas A, Deegan P et al (2017) Constitutive description of human femoropopliteal artery aging. Biomech Model Mechanobiol 16:681–692. https://doi.org/10.1007/s10237-016-0845-7
Keyes JT, Lockwood DR, Utzinger U et al (2013) Comparisons of planar and tubular biaxial tensile testing protocols of the same porcine coronary arteries. Ann Biomed Eng 41:1579–1591. https://doi.org/10.1007/s10439-012-0679-0
MacTaggart JN, Phillips NY, Lomneth CS et al (2014) Three-dimensional bending, torsion and axial compression of the femoropopliteal artery during limb flexion. J Biomech 47:2249–2256. https://doi.org/10.1016/j.jbiomech.2014.04.053
Mahoney EM, Wang K, Cohen DJ et al (2008) One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes 1:38–45. https://doi.org/10.1161/CIRCOUTCOMES.108.775247
Maleckis K, Anttila E, Aylward P et al (2018) Nitinol stents in the femoropopliteal artery: a mechanical perspective on material, design, and performance. Ann Biomed Eng. https://doi.org/10.1007/s10439-018-1990-1
Ogden RW (1997) Non-linear elastic deformations. Dover Publications, Mineola
Poulson W, Kamenskiy A, Seas A et al (2018) Limb flexion-induced axial compression and bending in human femoropopliteal artery segments. J Vasc Surg 67:607–613. https://doi.org/10.1016/j.jvs.2017.01.071
Saini A, Berry C, Greenwald S (1995) Effect of age and sex on residual stress in the aorta. J Vasc Res 32:398–405. https://doi.org/10.1159/000159115
Schillinger M, Sabeti S, Loewe C (2006) Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 354:1879–1888
Schillinger M, Sabeti S, Dick P et al (2007) Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 115:2745–2749. https://doi.org/10.1161/CIRCULATIONAHA.107.688341
Schulze-Bauer CAJ, Morth C, Holzapfel GA (2003) Passive biaxial mechanical response of aged human iliac arteries. J Biomech Eng 125:395. https://doi.org/10.1115/1.1574331
Sommer G, Holzapfel GA (2012) 3D constitutive modeling of the biaxial mechanical response of intact and layer-dissected human carotid arteries. J Mech Behav Biomed Mater 5:116–128. https://doi.org/10.1016/j.jmbbm.2011.08.013
Sommer G, Regitnig P, Koltringer L, Holzapfel GA (2010) Biaxial mechanical properties of intact and layer-dissected human carotid arteries at physiological and supra-physiological loadings. Am J Physiol Hear Circ Physiol 298:H898–H912
Sommer G, Benedikt C, Niestrawska JA et al (2017) Mechanical response of human subclavian and iliac arteries to extension, inflation and torsion. Acta Biomater 5:1. https://doi.org/10.1016/j.actbio.2018.05.043
Stålhand J, Klarbring A (2005) Aorta in vivo parameter identification using an axial force constraint. Biomech Model Mechanobiol 3:191–199. https://doi.org/10.1007/s10237-004-0057-4
Vaishnav RN, Vossoughi J (1983) Estimation of residual strains in aortic segments. In: Biomedical engineering II. Elsevier, pp 330–333
Valentín A, Humphrey JD, Holzapfel GA (2011) A multi-layered computational model of coupled elastin degradation, vasoactive dysfunction, and collagenous stiffening in aortic aging. Ann Biomed Eng 39:2027–2045. https://doi.org/10.1007/s10439-011-0287-4
Van Loon P, Klip W, Bradley E (1977) Length-force and volume-pressure relationships of arteries. Biorheology 14:181–201
Weizsacker H, Lambert H, Pascale K (1983) Analysis of the passive mechanical properties of rat carotid arteries. J Biomech 16(9):703–715
Acknowledgements
The authors wish to acknowledge Live On Nebraska for their help and support and thank tissue donors and their families for making this study possible.
Funding
Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01 HL125736.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in relation to this submission.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Cauchy stresses
The components of the isochoric Cauchy stress tensor \(\bar{\varvec{t}}\) associated with each of the residual, physiologic, and flattening deformations can be calculated by taking the appropriate derivatives in Eq. 3 and using a form of strain energy function defined by Eqs. 1 and 2. This produces Cauchy stresses in the form
with
where \(r, \theta , z\) are used to point out that these directions align with the radial, circumferential, and longitudinal directions of the artery in vivo. When considering specimen flattening, stretches \(\lambda_{r} , \lambda_{\theta } , \lambda_{z}\) need to be substituted with λ1, λ2, λ3. When considering residual deformations, they need to be substituted with \(\lambda_{\rho } , \lambda_{\vartheta } , \lambda_{\zeta }\). Finally, when considering physiologic deformations, these stretches need to be substituted with \(\lambda_{r}^{\text{phys}} ,\lambda_{\theta }^{\text{phys}} , \lambda_{z}^{\text{phys}}\).
1.2 Obtaining through-thickness values of stretch and stress
Through-thickness average values of stretch and stress were calculated by integrating them over the current volume and dividing the result by the total current volume. In Cartesian coordinates, since the stretches and the stresses vary through specimen thickness, this takes the form:
where L1 is the thickness of the flattened specimen. Similarly in the cylindrical coordinates:
1.3 Kinematics of the residual and physiologic deformations
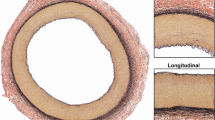
The stress-free (reference) configuration (Fig. 1a) is defined in terms of cylindrical coordinates \(\left( {R,\varTheta ,Z} \right)\) as
where Ri, Ro, α, and L are the inner and outer radii, opening angle, and the length of the stress-free arterial segment, respectively. The deformation gradient \(\varvec{F}_{\text{res}}\) takes this configuration into the load-free state (Fig. 1b) geometrically defined as
in which
In cylindrical coordinates, \(\varvec{F}_\text{res}\) is then given by
where \(\lambda_{\rho }\), λϑ, and λζ are the radial, circumferential, and longitudinal residual stretches, respectively, with λϑ given by (Ogden 1997; Holzapfel et al. 2000; Humphrey 2002; Sommer and Holzapfel 2012; Kamenskiy et al. 2014)
and \({\rm K} = \frac{2\pi }{2\pi - \alpha }\) is a measure of the circumferential opening angle. Assuming incompressibility, one can write
and similarly
By substituting R form Eq. 11 in Eq. 9, the circumferential residual stretch λϑ can be calculated at each point through thickness as a function of \(\rho ,R_{o} ,{\rm K}, \lambda_{\vartheta }^{o} ,\lambda_{\zeta }\), given by Eq. 6.
Further, deformation \(\varvec{F}_\text{load}\) takes the load-free configuration to the loaded (current) configuration (Fig. 1c) which in cylindrical coordinates is defined as
where
\(\varvec{F}_{\text{load}}\) is given by
in which \(\lambda_{\theta } = \frac{r}{\rho }\) is the circumferential stretch. Assuming incompressibility, similar to Eq. 11
The total deformation gradient with respect to the stress-free configuration is then given by
in which the circumferential stretch is calculated as
By substituting \(\rho_{o}^{2} - \rho^{2}\) from Eq. 10 in Eq. 12, one can find \(\lambda_{\theta }^\text{phys}\) as a function of \(r,R_{o} , {\rm K}, \lambda_{\vartheta }^{o} ,\lambda_{\zeta } ,\lambda_{z}\), given by Eq. 7
1.4 Equilibrium and boundary conditions
The total Cauchy stress in Eq. 3 (i.e., \(\varvec{t} = - p\varvec{I} + \bar{\varvec{t}}\)) contains both volumetric and isochoric parts (Ogden 1997; Humphrey 2002; Holzapfel and Ogden 2010a). Constitutive parameters define the isochoric stress tensor \(\bar{\varvec{t}}\) (Sect. 2.2), but the volumetric component \(- p\varvec{I}\) needs to be determined from the equilibrium and boundary conditions (Humphrey 2002; Holzapfel and Ogden 2010a).
Assuming quasi-static motions (Humphrey 2002), in the absence of body forces, for each of the deformations depicted in Fig. 1, the equilibrium equation is
where \(\text{div}\) represents the spatial divergence. Equation 13 can then be used to find the Lagrange multiplier \(p\) in Eq. 3, along with the unknown stretches.
For the deformation associated with the specimen flattening, Eq. 13 reduces to Ogden (1997) \(t_{11} = 0 ,\) and from Eq. 3, the volumetric part of the Cauchy stress can then be determined as \(p = \bar{t} _{11} .\)
In the load-free configuration, the only nontrivial component of Eq. 13 is (Humphrey 2002; Holzapfel and Ogden 2010a)
which reduces to
for \(t_{\rho \rho } \left( {\rho_{i} } \right) = 0\). The Lagrange multiplier can then be calculated at each point through the thickness of the artery by substituting tρρ into Eq. 3, i.e.,
In addition, since axial force vanishes in the load-free configuration due to release of longitudinal pre-stretch, the axial global equilibrium can be written as (Humphrey 2002; Holzapfel and Ogden 2010a)
where Fζ denotes the axial force. The above equation can be further reduced to (Humphrey 2002)
Equations 14 and 16 combined with the boundary condition \(t_{\rho \rho } \left( {\rho_{o} } \right) = 0\) are then solved together to calculate the unknown stretches \(\lambda_{\vartheta }^{o}\) and \(\frac{\xi }{L}\) in Eq. 6
Similar to Eq. 14, for the loaded configuration (Fig. 1c), one can write
where Pi is the internal luminal pressure, and the Lagrange multiplier is determined according to
Furthermore, assuming no pressure on the outer surface of the artery, one can find
which can be used to find \(\lambda_{\theta }^{o}\) in Eq. 7. Finally, the axial force in the loaded configuration can be calculated from (Humphrey 2002)
1.5 Variability of the mechanical properties within age groups
The four-fiber family constitutive parameters adjusted for the effects of flattening and representing the 25th and 75th percentiles within each age group are summarized in Tables 2 and 3, respectively.
Rights and permissions
About this article
Cite this article
Jadidi, M., Desyatova, A., MacTaggart, J. et al. Mechanical stresses associated with flattening of human femoropopliteal artery specimens during planar biaxial testing and their effects on the calculated physiologic stress–stretch state. Biomech Model Mechanobiol 18, 1591–1605 (2019). https://doi.org/10.1007/s10237-019-01162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01162-0