Abstract
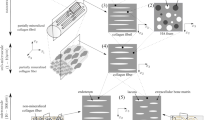
The key parameters influencing the elastic properties of the mineralized turkey leg tendon (MTLT) were investigated. Two structurally different tissue types appearing in the MTLT were considered: circumferential and interstitial tissue. These differ in their amount of micropores and their average diameter of the mineralized collagen fibril bundles. A multiscale model representing the apparent elastic stiffness tensor of MTLT tissue was developed using the Mori–Tanaka and the self-consistent homogenization schemes. The volume fraction of mineral (hydroxyapatite) in the fibril bundle, \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}\), and the tissue microporosity are the variables of the model. The MTLT model was analyzed performing a global sensitivity analysis (Elementary Effects method) and a parametric study. The stiffnesses parallel (axial) and perpendicular (transverse) to the MTLT long axis were the only significantly sensitive components of the apparent stiffness tensor of MTLT tissue. The most important parameters influencing these apparent stiffnesses are \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}\), tissue microporosity, as well as shape and distribution of the minerals in the fibril bundle (intra- vs. interfibrillar). The predicted apparent stiffness was converted to acoustic impedance for model validation. From measurements on embedded MTLT samples, including 50- and 200-MHz scanning acoustic microscopy as well as synchrotron radiation micro-computed tomography, we obtained site-matched acoustic impedance and \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}\) data of circumferential and interstitial tissue. The experimental and the model data compare very well for both tissue types (relative error 6–8 %).









Similar content being viewed by others
Abbreviations
- \(\text {axi}\) :
-
Axial direction (parallel to tendon long axis)
- \({\text {CIR}}\) :
-
Circumferential MTLT tissue
- \({\text {col}}\) :
-
Collagen
- \({\mathrm{DMB}}\) :
-
Degree of mineralization of bone
- \({\text {ES}}\) :
-
Extrafibrillar space
- \({\text {ha}}\) :
-
Hydroxyapatite
- \({\text {INT}}\) :
-
Interstitial MTLT tissue
- \({\text {MCF}}\) :
-
Mineralized collagen fibril
- \({\text {MCFB}}\) :
-
Mineralized collagen fibril bundle
- \({\text {MMT}}\) :
-
Musculoskeletal mineralized tissue
- \({\text {mp}}\) :
-
Micropores
- \({\text {MTLT}}\) :
-
Mineralized turkey leg tendon
- \({\text {np}}\) :
-
Nanopores
- \({\text {pmma}}\) :
-
Polymethylmethacrylate
- ROI :
-
Region of interest
- SAM:
-
Scanning acoustic microscopy
- SR-\(\mu \)CT:
-
Synchrotron radiation micro-computed tomography
- \(\text {trv}\) :
-
Transverse direction (perpendicular to tendon long axis)
- Subscript \(_i\) :
-
Subscript of quantities which relate to ROI \(i\)
- \(\nu , \nu _i\) :
-
Direction, transverse (trv) or axial (axi)
- \(T, T_i\) :
-
Type of tissue, CIR, INT, or CIR/INT
- \({\mathcal {S}}_{A}\) :
-
Model of composite material \(A\)
- \(C_A,\, C_{\nu }^{A}\) :
-
Apparent stiffness tensor of material \(A\); component of \(C_A\) in direction \(\nu \)
- \(Z^{A}_\nu \) :
-
Predicted acoustic impedance of material \(A\) in direction \(\nu \)
- \(\widetilde{Z}, \widetilde{Z}_{i}\) :
-
Experimental acoustic impedance
- \({\mathrm{DMB}}, {\mathrm{DMB}}_i\) :
-
Experimental DMB
- \(\mathrm{{\mathrm{DMB}}}^{{\text {MCFB}}}\) :
-
DMB of MCFB tissue
- \(\hbox {vf}_B^A\) :
-
Volume fraction of material \(B\) in (composite) material \(A\)
- \(\widetilde{\hbox {vf}}_{{\text {mp}}}\) :
-
Experimental microporosity of MTLT tissue derived from light microscopy images
- \(\hbox {ar}_A\) :
-
Aspect ratio of a spheroidal inclusion made of material \(A\)
- \(\text {rRMSE}_{\nu }^T\) :
-
Relative root mean square error of the predicted acoustic impedance w.r.t. experimentally assessed values for tissue type \(T\) in direction \(\nu \)
- \(\mathrm{rME}_{\nu }^T\) :
-
Maximum relative error of the predicted acoustic impedance w.r.t. a linear regression model of the experimental data for tissue type \(T\) in direction \(\nu \)
References
Akkus O (2005) Elastic deformation of mineralized collagen fibrils: an equivalent inclusion based composite model. J Biomech Eng 127(3):383–390
Alexander B, Daulton TL, Genin GM, Lipner J, Pasteris JD, Wopenka B, Thomopoulos S (2012) The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen-mineral structure. J R Soc Interface 9(73):1774–1786
Eshelby JD (1957) The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc R Soc Lond A Math Phys Sci 241(1226):376–396
Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK (2005) Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 4:612–616
Fratzl P (2008) Collagen: structure and mechanics. Springer, Berlin
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52(8):1263–1334
Fritsch A, Hellmich C (2007) ’Universal’ microstructural patterns in cortical and trabecular, extracellular and extravascular bone materials: micromechanics-based prediction of anisotropic elasticity. J Theor Biol 244(4):597–620
Granke M, Gourrier A, Rupin F, Raum K, Peyrin F, Burghammer M, Saïed A, Laugier P (2013) Microfibril orientation dominates the microelastic properties of human bone tissue at the lamellar length scale. PLoS ONE 8(3):58043
Grimal Q, Raum K, Gerisch A, Laugier P (2011) A determination of the minimum sizes of representative volume elements for the prediction of cortical bone elastic properties. Biomech Model Mechanobiol 10(6):925–937
Hamed E, Lee Y, Jasiuk I (2010) Multiscale modeling of elastic properties of cortical bone. Acta Mech 213(1–2):131–154
Hellmich C, Barthélémy JF, Dormieux L (2004) Mineral–collagen interactions in elasticity of bone ultrastructure: a continuum micromechanics approach. Eur J Mech A Solids 23(5):783–810
Hill R (1965) A self-consistent mechanics of composite materials. J Mech Phys Solids 13(4):213–222
Jäger I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79(4):1737–1746
Lakshmanan S, Bodi A, Raum K (2007) Assessment of anisotropic tissue elasticity of cortical bone from high-resolution, angular acoustic measurements. IEEE Trans Ultrason Ferroelectr Freq Control 54(8):1560–1570
Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF (1993) Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol 110(1):39–54
Lees S, Prostak KS, Ingle VK, Kjoller K (1994) The loci of mineral in turkey leg tendon as seen by atomic force microscope and electron microscopy. Calcif Tissue Int 55(3):180–189
Malo MKH, Rohrbach D, Isaksson H, Töyräs J, Jurvelin JS, Tamminen IS, Kröger H, Raum K (2013) Longitudinal elastic properties and porosity of cortical bone tissue vary with age in human proximal femur. Bone 53(2):451–458
Mori T, Tanaka K (1973) Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall 21(5):571–574
Morris MD (1991) Factorial sampling plans for preliminary computational experiments. Technometrics 33(2):161–174
Nair AK, Gautieri A, Chang SW, Buehler MJ (2013) Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun 4:1724
Nikolov S, Raabe D (2008) Hierarchical modeling of the elastic properties of bone at submicron scales: the role of extrafibrillar mineralization. Biophys J 94(11):4220–4232
Nuzzo S, Peyrin F, Cloetens P, Baruchel J, Boivin G (2002) Quantification of the degree of mineralization of bone in three dimensions using synchrotron radiation microtomography. Med Phys 29(11):2672–2681
Raum K (2008) Microelastic imaging of bone. IEEE Trans Ultrason Ferroelectr Freq Control 55(7):1417–1431
Raum K (2011) Microscopic elastic properties. In: Laugier P, Haïat G (eds) Bone quantitative ultrasound, chap 16, 1st edn. Springer, Berlin, pp 409–440
Raum K, Cleveland RC, Peyrin F, Laugier P (2006a) Derivation of elastic stiffness from site-matched mineral density and acoustic impedance maps. Phys Med Biol 51(3):747–758
Raum K, Leguerney I, Chandelier F, Talmant M, Saïed A, Peyrin F, Laugier P (2006b) Site-matched assessment of structural and tissue properties of cortical bone using scanning acoustic microscopy and synchrotron radiation \(\mu \)CT. Phys Med Biol 51(3):733–746
Raum K, Hofmann T, Leguerney I, Saïed A, Peyrin F, Vico L, Laugier P (2007) Variations of microstructure, mineral density and tissue elasticity in B6/C3H mice. Bone 41(6):1017–1024
Raum K, Laugier P, Grimal Q, Gerisch A (2011) Multiscale structure-functional modeling of lamellar bone. Proc Meet Acoust 9(1):020005
Reisinger AG, Pahr DH, Zysset PK (2010) Sensitivity analysis and parametric study of elastic properties of an unidirectional mineralized bone fibril-array using mean field methods. Biomech Model Mechanobiol 9(5):499–510
Rohrbach D, Lakshmanan S, Peyrin F, Langer M, Gerisch A, Grimal Q, Laugier P, Raum K (2012) Spatial distribution of tissue level properties in a human femoral cortical bone. J Biomech 45(13):2264–2270
Rupin F, Saïed A, Dalmas D, Peyrin F, Haupert S, Raum K, Barthel E, Boivin G, Laugier P (2009) Assessment of microelastic properties of bone using scanning acoustic microscopy: a face-to-face comparison with nanoindentation. Jpn J Appl Phys 48(7):07GK01
Saltelli A, Ratto M, Andres T, Campolongo F, Cariboni J, Gatelli D, Saisana M, Tarantola S (2008) Global sensitivity analysis: the primer. Wiley, New York
Sasaki N, Tagami A, Goto T, Taniguchi M, Nakata M, Hikichi K (2002) Atomic force microscopic studies on the structure of bovine femoral cortical bone at the collagen fibril-mineral level. J Mater Sci Mater Med 13(3):333–337
Spiesz EM, Roschger P, Zysset PK (2012a) Elastic anisotropy of uniaxial mineralized collagen fibers measured using two-directional indentation. Effects of hydration state and indentation depth. J Mech Behav Biomed Mater 12:20–28
Spiesz EM, Roschger P, Zysset PK (2012b) Influence of mineralization and microporosity on tissue elasticity: experimental and numerical investigations on mineralized turkey leg tendons. Calcif Tissue Int 90(4):319–329
Tandon GP, Weng GJ (1984) The effect of aspect ratio of inclusions on the elastic properties of unidirectionally aligned composites. Polym Compos 5(4):327–333
Vaughan TJ, McCarthy CT, McNamara L (2012) A three-scale finite element investigation into the effects of tissue mineralisation and lamellar organisation in human cortical and trabecular bone. J Mech Behav Biomed Mater 12:50–62
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28:271–298
Zaoui A (2002) Continuum micromechanics: survey. J Eng Mech 128(8):808–816
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft (SPP 1420 Grants RA 1380/7, GE 1894/3) and has been conducted within the European Associated Laboratory “Ultrasound Based Assessment of Bone” (ULAB).
Appendix
Appendix
1.1 Phase volume fractions
We give below the complete set of formulas that determine the phase volume fractions of our model sequence.
1.1.1 MTLT tissue
The phase volume fractions of the composite MTLT (CIR or INT tissue) are given by:
1.1.2 MCFB
The phase volume fractions of the MCFB are given by:
The function \(h\) is derived based on an empirical formula of (Raum et al. (2006a), Eq. (10), p. 750), which connects the collagen volume fraction \(\hbox {vf}_{{\text {col}}}^{{\text {MCFB}}}\), the mineral volume fraction \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}\) and the nanopores volume fraction \(\hbox {vf}_{{\text {np}}}^{{\text {MCFB}}}\) to each other:
Since ha, col and np are the sole basic constituents of the MCFB, their corresponding volume fractions \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}, \hbox {vf}_{{\text {col}}}^{{\text {MCFB}}}\) and \(\hbox {vf}_{{\text {np}}}^{{\text {MCFB}}}\) sum up to one. We then eliminate \(\hbox {vf}_{{\text {np}}}^{{\text {MCFB}}}\) from Eq. (17) using \(\hbox {vf}_{{\text {np}}}^{{\text {MCFB}}} = 1-\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}- \hbox {vf}_{{\text {col}}}^{{\text {MCFB}}}\) and obtain
The function \(h\) is defined by the right-hand side of the equation.
The mineral volume fraction \(\hbox {vf}_{{\text {ha}}}^{{\text {MCFB}}}\) is given by
where \(\beta \in [0,\widetilde{\hbox {vf}}_{{\text {mp}}}]\) is the amount of microporosity which influences the DMB measurements. \(\rho _{{\text {ha}}}\) is the mass density of ha and \(\mathrm {{\mathrm{DMB}}}\) is the experimental DMB value.
1.1.3 MCF
The phase volume fractions of the MCF are given by:
1.1.4 ES
The phase volume fractions of the ES are given by:
Rights and permissions
About this article
Cite this article
Tiburtius, S., Schrof, S., Molnár, F. et al. On the elastic properties of mineralized turkey leg tendon tissue: multiscale model and experiment. Biomech Model Mechanobiol 13, 1003–1023 (2014). https://doi.org/10.1007/s10237-013-0550-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0550-8