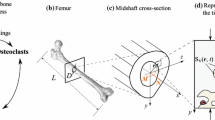
Abstract
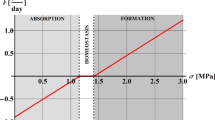
Bone as most of living tissues is able, during its entire lifetime, to adapt its internal microstructure and subsequently its associated mechanical properties to its specific mechanical and physiological environment in a process commonly known as bone remodelling. Bone is therefore continuously renewed and microdamage, accumulated by fatigue or creep, is removed minimizing the risk of fracture. Nevertheless, bone is not always able to repair itself completely. Actually, if bone repairing function is slower than microdamage accumulation, a type of bone fracture, usually known as “stress fracture”, can finally evolve. In this paper, we propose a bone remodelling continuous model able to simulate microdamage growth and repair in a coupled way and able therefore to predict the occurrence of “stress fractures”. The biological bone remodelling process is modelled in terms of equations that describe the activity of basic multicellular units. The predicted results show a good correspondence with experimental and clinical data. For example, in disuse, bone porosity increases until an equilibrium situation is achieved. In overloading, bone porosity decreases unless the damage rate is so high that causes resorption or “stress fracture”.

























Similar content being viewed by others
References
Wolff J (1986) The Law of Bone Remodelling Das Gesetz der Transformation der Knochen, Kirschwald, 1892 Translated by Maquet P, Furlong R. Springer, Berlin
Cowin SC (2001) The false premise in Wolff’s Law. In: Bone mechanics handbook, 2nd edn, chap. 30, CRC Press, Boca Raton, pp 30-1–30-15
Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Sloof TJ (1987) Adaptive bone-remodelling theory applied to prosthetic-design analysis. J Biomech 20(11/12):1135–1150
Carter DR, Fyhrie DP, Whalen RT (1987) Trabecular bone density and loading history: regulation of tissue biology by mechanical energy. J Biomech 20:785–795
Carter DR, Orr TE, Fyhrie DP (1989) Relationships between loading history and femoral cancellous bone architecture. J Biomech 22(3):231–244
Prendergast PJ, Taylor D (1994) Prediction of bone adaptation using damage accumulation. J Biomech 27:1067–1076
Martin RB (1995) A mathematical model for fatigue damage repair and stress fracture in osteonal bone. J Orthop Res 13:309–316
Jacobs CR, Simo JC, Beaupré GS, Carter DR (1997) Adaptive bone remodeling incorporating simultaneous density and anisotropy considerations. J Biomech 30(6):603–613
Hart RT, Fritton SP (1997) Introduction to finite element based simulation of functional adaptation of cancellous bone. Forma 12:277–299
Fernandes P, Rodrigues H, Jacobs CR (1999) A model of bone adaptation using a global optimisation criterion based on the trajectorial theory of wolff. Comput Methods Biomech Biomed Engin 2(2):125–138
Doblaré M, García JM (2002) Anisotropic bone remodelling model based on a continuum damage-repair theory. J Biomech 35(1):1–17
Doblaré M, García JM (2001) Application of an anisotropic bone-remodelling model based on a damage-repair theory to the analysis of the proximal femur before and after total hip replacement. J Biomech 34(9):1157–1170
Cowin SC, Hegedus DH (1976) Bone remodeling i: a theory of adaptive elasticity. J Elasticity 6:313–326
Hart RT, Davy DT, Heiple KG (1984) A computational model for stress analysis of adaptive elastic materials with a view toward applications in strain-induced bone remodelling. J Biomech Engin 106:342–350
Beaupré GS, Orr TE, Carter DR (1990) An approach for time-dependent bone modeling and remodeling-theoretical development. J Orthop Res 8(5):651–661
Weinans H, Huiskes R, Grootenboer HJ (1992) The behavior of adaptive bone-remodeling simulation models. J Biomech 25:1425–1441
Cowin SC (1986) Wolff’s law of trabecular architecture at remodeling equilibrium. J Biomech Eng 108(1):83–88
Luo GM, Cowin SC, Sadegh AM (1992) An evolutionary wolff’s law for trabecular architecture. J Biomech Eng 114(1):129–136
Ramtani S, Zidi M (2001) A theoretical model of the effect of continuum damage on a bone adaptation model. J Biomech 34(4):471–479
Doblaré M, Ramtani S, García JM (2004) Computer simulation of an adaptive damage-bone remodeling law applied to three unit-bone bars structure. Comput Biol Med 34(3):259–273
Hart RT (2001) Bone modeling and remodeling: theories and computation. In: Bone mechanics handbook, 2nd edn, chap 31, CRC Press, Boca Raton, pp 31-1–31-42
Petermann HE, Reiter TJ, Rammerstorfer FG (1997) Computational simulation of internal bone remodeling. Arch Comput Meth Eng 4(4):295–323
Huiskes R, Ruimerman R, GH van Lenthe, Janssen JD (2000) Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704–706
Hazelwood SJ, Martin RB, Rashid MM, Rodrigo JJ (2001) A mechanistic model for internal bone remodeling exhibits different dynamic responses in disuse and overload. J Biomech 34:299–308
Hernandez CJ (2001) Simulation of bone remodeling during the development and treatment of osteoporosis. PhD thesis, Stanford University, Stanford, CA
Hernandez CJ, Beaupré GS, Carter DR (2000) A model of mechanobiologic and metabolic influences on bone adaptation. J Rehabil Res Dev 37(2):235–244
Taylor D, Lee TC (2003) Microdamage and mechanical behaviour: predicting failure and remodelling in compact bone. J Anat 203:203–211
Taylor D, Casolari E, Bignardi C (2004) Predicting stress fractures using a probabilistic model of damage, repair and adaptation. J Orthop Res 22(3):487–494
Hernandez CJ, Beaupré GS, Marcus R, Carter DR (2001) A theoretical analysis of the contributions of remodeling space, mineralization, and bone balance to changes in bone mineral density during alendronate treatment. Bone 29(6):511–516
Hernandez CJ, Beaupré GS, Keller TS, Carter DR (2001) The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone 29(1):74–78
Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R (1998) Does microdamage accumulation affect the mechanical properties of bone? J Biomech 31(4):337–345
Jepsen KJ, Davy DT, Akkus O (2001) Observations of damage in bone. In: Bone mechanics handbook, 2nd edn, chap 17, CRC Press, Boca Raton, pp 17-1–17-18
Davy DT, Jepsen KJ (2001) Bone damage mechanics. In: Bone mechanics handbook, 2nd edn. chap 18, CRC Press, Boca Raton, pp 18-1–18-25
Pattin CA, Caler WE, Carter DR (1996) Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech 29(1):69–79
Zioupos P, Currey JD (1998) Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 22(1):57–66
Lemaitre J, Chaboche JL (1990) Mechanics of solid materials. Cambridge University Press, Cambridge
Simo JC, Ju JW (1987) Strain and stress-based continuum damage models: formulation. Int J Solids Struct 23(7):821–840
Keller TS (1994) Predicting the compressive mechanical behavior of bone. J Biomech 27(9):1159–1168
Martin RB, Burr DR, Sharkey NA (1998) Skeletal tissue mechanics. Springer, Berlin Heidelberg New York
Frost HM (1964) Dynamics of bone remodelling. In: Bone biodynamics. Little Brown Co, Boston, pp 315–333
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Low mechanical signals strengthen long bones. Nature 412:603–604
Carter DR, Beaupré GS (2001) Skeletal function and form. Cambridge University Press, Cambridge
Mikic B, Carter DR (1995) Bone strain gage data and theoretical models of functional adaptation. J Biomech 28(4):465–469
Whalen RT, Carter DR, Steele CR (1988) Influence of physical activity on the regulation of bone density. J Biomech 21(10):825–837
Jacobs CR (1994) Numerical simulation of bone adaptation to mechanical loading. PhD thesis, Stanford University, Stanford, CA
Carter DR (1984) Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int 36:S19–S24
Tsubota K, Adachi T, Tomita Y (2002) Functional adaptation of cancellous bone in human proximal femur predicted by trabecular surface remodeling simulation toward uniform stress state. J Biomech 35(12):1541–1551
Turner CH (1999) Toward a mathematical description of bone biology: the principle of cellular accommodation. Calcif Tissue Int 65(6):466–471
Zioupos P, Currey JD (1998) Cumulative damage and the response of human bone in two-step loading fatigue. J Biomech 31:825–833
Lee TC, FJ O’Brien, Taylor D (2000) The nature of fatigue damage in bone. Int J Fatigue 22:847–853
Parfitt AM (1983) The physiologic and clinical significance of bone hisotomorphometric data. In: Bone histomorphometry techniques and interpretation. CRC Press, Boca Raton, pp 143–223
Martin RB (1984) Porosity and specific surface of bone. In: Critical reviews in biomedical engineering, vol 10, chap 3, CRC Press, Boca Raton, pp 179–222
Gross TS, Rubin CT (1995) Uniformity of resorptive bone loss induced by disuse. J Orthop Res 13(5):708–714
Li XJ, Jee WS, Chow SY, Woodbury DM (1990) Adaptation of cancellous bone to aging and immobilization in the rat: a single photon absorptiometry and histomorphometry study. Anat Rec 227(1):12–24
Takata S, Yasui N (2001) Disuse osteoporosis. J Med Invest 48(3-4):147–156
ZFG Jaworski, Uhthoff HK (1986) Reversibility of nontraumatic disuse osteoporosis during its active phase. Bone 7:431–439
Martin RB (2003a) Fatigue microdamage as an essential element of bone mechanics and biology. Calcif Tissue Int 73(2):101–107
Martin RB (2003b) Fatigue damage, remodeling, and the minimization of skeletal weight. J Theor Biol 220(2):271–276
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB (1990) Intracortical remodeling in adult rat long bones after fatigue loading. J Orthop Res 8(5):651–661
Mori S, Burr DB (1993) Increased intracortical remodeling following fatigue damage. Bone 14(2):103–109
Verbogt O, Gibson GJ, Schaffler MB (2000) Loss of osteocyte integrity in association with microdamage and bone remodelling after fatigue in vivo. J Bone Miner Res 15:60–67
Martin RB (2000) Toward a unifying theory of bone remodeling. Bone 26(1):1–6
Cowin SC, Moss ML (2001) Mechanosensory mechanisms in bone. In: Bone mechanics handbook, 2nd edn, chap 29, CRC Press, Boca Raton, pp 29-1–29-17
Noble B (2003) Bone microdamage and cell apoptosis. Eur Cell Mater 21(6):46–55
Taylor D, Hazenberg JG, Lee TC (2003) The cellular transducer in damage-stimulated bone remodelling: a theoretical investigation using fracture mechanics. J Theor Biol 225(1):65–75
Frost HM (1969) Tetracycline-based histological analysis of bone remodelling. Calcif Tissue Res 3:211–237
Maloney WJ, Schmalzried T, Harris WH (2002) Analysis of long-term cemented total hip arthroplasty retrievals. Clin Orthop 405:70–78
Venesmaa PK, Kroger HP, Jurvelin JS, Miettinen HJ, Suomalainen OT, and Alhava EM (2003) Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand 74(1):31–36
Hernandez CJ, GS Beaupré, Carter DR (2003) A theoretical analysis of the changes in basic multicellular unit activity at menopause. Bone 32:357–363
Currey JD (1995) The validation of algorithms use to explain adaptive remodeling in bone. In: Bone structure and remodeling. World Scientific, Singapore, pp 9–13
Currey JD (2002) Bones Structure and mechanics. Princeton University Press, New Jersey
Parfitt AM (1996) Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. In: Osteoporosis, vol 1. Academic Press, New York
Komarova SV, Smith RJ, Dixon SJ, Sims SM, Wahl LM (2003) Mathematical model predicts a critical role for osteoclast autocrine regulation in the control of bone remodeling. Bone 33(2):206–215
Lane NE, Sanchez S, Modin GW (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. J Clin Invest 102:1627–1633
Hazelwood SJ, Martin RB, Nyman JS, Yeh OC (2004) A theoretical analysis of long-term biphosphonate effects on trabecular bone volume and microdamage. Bone 35:296–305
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res Oct 10(10):1478–1487
Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF (1996) A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res 11(2):150–159
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21(2):115–137
Author information
Authors and Affiliations
Corresponding author
Additional information
Research partially supported by Diputación General de Aragón project P–008/2001) and National Network IM3 (Molecular and Multimodal Medical Imaging, Spanish Ministry of Health, Associated Partner, 300++, 2003-2005)
Rights and permissions
About this article
Cite this article
García-Aznar, J.M., Rueberg, T. & Doblare, M. A bone remodelling model coupling microdamage growth and repair by 3D BMU-activity. Biomech Model Mechanobiol 4, 147–167 (2005). https://doi.org/10.1007/s10237-005-0067-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-005-0067-x