Abstract
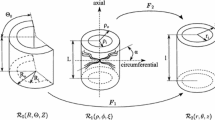
This paper addresses the problem of constructing a mechanical model for the abdominal aorta and calibrating its parameters to in vivo measurable data. The aorta is modeled as a pseudoelastic, thick-walled, orthotropic, residually stressed cylindrical tube, subjected to an internal pressure. The model parameters are determined by stating a minimization problem for the model pressure and computing the optimal solution by a minimization algorithm. The data used in this study is in vivo pressure–diameter data for the abdominal aorta of a 24-year-old man. The results show that the axial, circumferential and radial stresses have magnitudes in the span 0 to 180 kPa. Furthermore, the results show that it is possible to determine model parameters directly from in vivo measurable data. In particular, the parameters describing the residual stress distribution can be obtained without interventional procedures.
















Similar content being viewed by others
References
Antman SS (1995) Nonlinear problems of elasticity. Springer, Berlin Heidelberg New York
Burton AC (1954) Relation of structures to function of the tissues of the wall of blood vessels. Physiol Rev 34:619–642
Choung CJ, Fung YC (1986) On residual stress in arteries. J Biomech Eng 108:189–192
Fung YC (1993) Biomechanics: mechanical properties of living tissue, 2nd edn. Springer, Berlin Heidelberg New York
Gurtin ME (1982) Introduction to continuum mechanics. Academic, San Diego
Holzapfel GA, Weizsäcker HW (1998) Biomechanical behavior of the arterial wall and its numerical characterization. Comput Biol Med 28:377–392
Holzapfel GA (2000) Nonlinear solid mechanics. A continuum approach for engineering. Wiley, Chichester
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Holzapfel GA, Gasser TC (2001) A viscoelastic model for fiber-reinforced composites at finite strains. Comput Meth Appl Mech Eng 190:4379–4430
Holzapfel GA, Gasser TC, Stadler M (2002) A structural model for the viscoelastic behavior of arterial walls: continuum based formulation and finite element analysis. Eur J Mech A Solid 21:441–463
Humphrey JD (2002) Cardiovascular solid mechanics. Springer, Berlin Heidelberg New York
Patel DJ, Fry DL (1969) The elastic symmetry of arterial segments in dogs. Circ Res 24:1–8
Rachev A, Hayashi K (1999) Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distribution in arteries. Ann Biomed Eng 27:459–468
Rhodin JAG (1980) Architecture of the vessel wall. In: Bohr DF, Somlyo AD, Sparks HV (eds) Handbook of physiology, the cardiovascular system, sect 2, vol 2. American Physiological Society, Bethesda, MD, USA, pp 1–31
Schulze-Bauer CAJ, Holzapfel GA (2003) Determination of constitutive equations for human arteries from clinical data. J Biomech 36:165–169
Schulze-Bauer CAJ, Regitnig P, Holzapfel GA (2002) Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol 282:H2427–H2440
Sonneson B, Hansen F, Stale H, Länne T (1993) Compliance and diameter in the human abdominal aorta. Eur J Vasc Sur 7:690–697
Sonneson B, Länne T, Vernersson E, Hansen F (1994) Sex difference in the mechanical properties of the abdominal aorta in human beings. J Vasc Surg 20:959–969
Summa Y (1978) Determination of the tangential elastic modulus of human arteries in vivo. In: Bauer RD, Busse R (eds) The arterial system: dynamics, control theory and regulation. Springer, Berlin, pp 95–100
Takamizawa K, Hayashi K (1987) Strain energy density function and uniform strain hypothesis for arterial mechanics. J Biomech 20:7–17
von Maltzahn WW, Keitzer WF (1984) Experimental measurments of elastic properties of media and adventitia of bovine carotid arteries. J Biomech 17:839–847
Vorp DA, Rajagopal KR, Smolinski PJ, Borovetz HS (1995) Identification of elastic properties of homogeneous, orthotropic vascular segments in distension. J Biomech 28:501–512
Warne DP, Warne PG (1999a) Plane deformations in incompressible nonlinear elasticity. J Elast 52:129–158
Warne DP, Warne PG (1999b) Errata—plane deformations in incompressible nonlinear elasticity. J Elast 55:239
Yin FCP, Chew PH, Zeger SL (1986) An apporach to quantification of biaxial tissue stress-strain data. J Biomech 19:27–37
Acknowledgements
This work was financially supported by the National Graduate School in Scientific Computing (NGSSC). The authors wish to thank Professor Toste Länne at the Department for Medicine and Care at Linköping University for his invaluable help throughout this project, and for supplying in vivo data.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Equilibrium conditions in cylindrical coordinates are well known and are presented in standard textbooks when written in terms of the Cauchy stress. However, we use the first Piola–Kirchhoff stress and two sets of base vectors as explained in the section on deformation. Therefore, an explicit derivation of Eqs. (7), (8) and (9) seems appropriate.
The divergence of a tensor T, denoted divT is defined by
for any constant vector c. The left-hand side of Eq. (32) is the divergence of a vector field and is assumed well defined.
Taking an arbitrary vector c = c i j i ( αφ) we may write
Thus, T iK c i are the components of the vector T T c in cylindrical coordinates. The divergence of a vector in such coordinates is well known, and we have from the definition of divergence of a tensor in Eq. (32) that
Since c is constant we have
Furthermore, one concludes from definitions of base vectors
which we introduce into Eq. (33) and use
to obtain
Since c is arbitrary Eqs. (7), (8) and (9) now follow. For a different derivation of these equations, see Warne and Warne (1999a, 1999b).
Rights and permissions
About this article
Cite this article
Stålhand, J., Klarbring, A. & Karlsson, M. Towards in vivo aorta material identification and stress estimation. Biomech Model Mechanobiol 2, 169–186 (2004). https://doi.org/10.1007/s10237-003-0038-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-003-0038-z