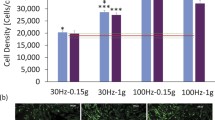
Abstract
In adaptive bone remodeling, mechanical signals such as stress/strain caused by loading/deformation are believed to play important roles as regulators of the process in which osteoclastic resorption and osteoblastic formation are coordinated under a local mechanical environment. The mechanism by which cells sense and transduce mechanical signals to the intracellular biochemical signaling cascade is still unclear, however to address this issue, the present study investigated the characteristic response of a single osteoblastic cell, MC3T3-E1, to a well-defined mechanical stimulus and the involvement of the cytoskeletal actin fiber structure in the mechanotransduction pathway. First, by mechanically perturbing to a single cell using a microneedle, a change in the intracellular calcium ion concentration [Ca2+]i was observed as a primal signaling response to a mechanical stimulus, and the threshold value of the perturbation as the mechanical stimulus was evaluated quantitatively. Second, to study directional dependence of the response to the mechanical stimulus, the effect of actin fiber orientation on the threshold value of the calcium response was investigated at various magnitudes and directions of the stimulus. It was found that the osteoblastic response to the perturbation exhibited a directional dependence. That is, the sensitivity of osteoblastic cells to a mechanical stimulus depends on the angle of the applied deformation with respect to the cytoskeletal actin fiber orientation. This finding is phenomenological evidence that cytoskeletal actin fiber structures are involved in the mechanotransduction mechanism, which may be related to cell polarization behaviors such as cellular alignment caused by mechanical stimulation.










Similar content being viewed by others
References
Adachi T, Murai T, Hoshiai S, Tomita T (2001) Effect of actin filament on deformation-induced Ca2+ response in osteoblast-like cells. Int J JSME Ser C 44:914–919
Banes AJ, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L (1995) Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signal. Biochem Cell Biol 73:349–365
Buckley MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, Gilbert J, Link GW, Tran Son Tay R (1988) Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner 4:225–236
Cowin SC (1985) The relationship between the elasticity tensor and the fabric tensor. Mech Mater 4:137–147
Cowin SC, Moss-Salentijn L, Moss ML (1991) Candidates for the mechanosensory system in bone. Trans ASME, J Biomech Eng 113:191–197
Donahue HJ, McLeod KJ, Rubin CT, Andersen J, Grine EA, Hertzberg EL, Brink PR (1995) Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res 10:881–889
Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tiss Int 57:344–358
Frost HM (1987) The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2:73–85
Guilak F, Donahue HJ, Zell R, Grande DA, McLeod KJ, Rubin CT (1994) Deformation-induced calcium signaling in articular chondrocytes. In: van Mow C, Guilak F, Tran-Son-Tay R, Hochmuth RM (eds) Cell mechanics and cellular engineering. Springer-Verlag, Berlin Heidelberg New York, pp 380–397
Guharay F, Sachs F (1984) Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352:685–701
Harell A, Dekel S, Binderman I (1977) Biochemical effect of mechanical stress on cultured bone cells. Calcif Tiss Res Suppl 22:202–207
Hasegawa S, Sato S, Saito S, Suzuki Y, Brunette DM (1985) Mechanical stretching increases the number of cultured bone cells synthesizing DNA and alters their pattern of protein synthesis. Calcif Tiss Int 37:431–436
Ingber D (1991) Integrins as mechanochemical transducers. Curr Opin Cell Biol 3:841–848
Jacob CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ (1998) Differential effect of steady versus oscillating flow on bone cells. J Biomech 31:969–976
Jørgensen NR, Geist ST, Civitelli R, Steinberg TH (1997) ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 139:497–506
Kurata K, Uemura T, Nemoto A, Tateishi T, Murakami T, Higaki H, Miura H, Iwamoto Y (2001) Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res 16:722–730
Levesque MJ, Nerem RM (1985) The elongation and orientation of cultured endothelial cells in response to shear stress. Trans ASME, J Biomech Eng 107:341–347
Meazzini MC, Toma CD, Schaffer JL, Gray ML, Gerstenfeld LC (1998) Osteoblast cytoskeletal modulation in response to mechanical strain in vitro. J Orthop Res 16:170–180
Meyer CJ, Alenghat FJ, Rim P, Fong JH-J, Fabry B, Ingber DE (2000) Mechanical control of cyclic AMP signaling and gene transcription through integrins, Nature Cell Biol 2:666–668
Naruse K, Yamada T, Sokabe M (1998) Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol 274–5:H1532–1538
Neidlinger-Wilke C, Wilke HJ, Claes L (1994) Cyclic stretching of human osteoblasts affects proliferation and metabolism: a new experimental method and its application. J Orthop Res 12:70–78
Neidlinger-Wilke C, Grood ES, Wang JH-C, Brand RA, Claes L (2001) Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J Orthop Res 19:286–293
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tiss Int 37:411–417
Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, Antonova GN, Smirnov VN (1989) Mechano-chemical control of human endothelium orientation and size. J Cell Biol 109:331–339
Sokabe M, Sachs F, Jing Z-Q (1991) Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J 59:722–728
Sokabe M, Naruse K, Sai S, Yamada T, Kawakami K, Inoue M, Murase K, Miyazu M (1997) Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels Suppl 12:191–193.
Turner CH, Pavalko FM (1998) Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3:346–355
van der Meulen MCH, Huiskes R (2001) Why mechanobiology? A survey article. J Biomech 35:401–414
Wang JH-C, Grood ES, Florer J, Wenstrup R (2000) Alignment and proliferation of MC3T3-E1 osteoblasts in microgrooved silicone substata subjected to cyclic stretching. J Biomech 33: 729–735
Wang JH-C, Goldschmidt-Clermont P, Wille J, Yin FC (2001a) Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 34:1563–1572
Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE (2001b) Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA 98:7765–7770
Wolff J (1892) Das Gesetz der Transformation der Knochen (The law of bone remodeling, trans. by Maquet P, Furlong R). Springer-Verlag, Heidelberg Berlin New York
Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA (2000) Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of α V β 3-integrin expression. J Bone Miner Res 15:1731–1745
Xia S-L, Ferrier J (1992) Propagation of a calcium pulse between osteoblastic cells. Biochem Biophys Res Commun 186:1212–1219
Yeh C-K, Rodan GA (1984) Tensile forces enhance prostaglandin E synthesis in osteoblastic cells grown on collagen ribbons. Calcif Tiss Int Suppl 36:S67–S71
Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ (2000) Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res 15:209–217
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adachi, T., Sato, K. & Tomita, Y. Directional dependence of osteoblastic calcium response to mechanical stimuli. Biomech Model Mechanobiol 2, 73–82 (2003). https://doi.org/10.1007/s10237-003-0029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-003-0029-0