Abstract
We conducted jar tests on the efficacy of using various mixtures of pulverized bentonite clay and Parrotia persica plant to treat AMD from a mine in Mogale City, South Africa without adding alkalinity. Two nearly identical sets of experiments were conducted using the same materials after they were rinsed using distilled and potable waters, respectively, to remove impurities, and then dried in an oven at 60 °C for 24 h, after which they were pulverized again. The pH, electrical conductivity, oxidation reduction potential, turbidity, and metal concentrations were measured before and after the experiments. The samples were treated in a jar test using standard procedures, i.e. rapid and slow mixing and settling for 1 h. The results showed that increasing the concentration of P. persica relative to the bentonite improved treatment effectiveness and that the highest turbidity removal (> 96.8%) was produced by rinsing the mixtures with potable water, drying them, and then pulverizing them again. Potentially toxic metals were removed by more than 93.7%. The morphological structure of the SEM micrographs indicate that the removal mechanism involves both physical and chemical phenomena and that the clay and P. persica are both important, particularly to the physical aspects.
Zusammenfassung
Wir untersuchten die Wirksamkeit verschiedener Mischungen aus pulverisiertem Bentonit-Ton und Parrotia persica zur Behandlung von saurem Grubenwasser aus einer Mine in Mogale City, Südafrika, ohne Zugabe von Alkalinität. Zwei nahezu identische Versuchsreihen wurden mit denselben Materialien durchgeführt, nachdem sie mit destilliertem Wasser bzw. Trinkwasser gespült worden waren, um Verunreinigungen zu entfernen, und dann 24 Stunden lang bei 60 °C im Ofen getrocknet und anschließend erneut pulverisiert worden waren. Vor und nach den Experimenten wurden der pH-Wert, die Leitfähigkeit, das Oxidations-Reduktionspotenzial, die Trübung und die Konzentration toxischer Metalle gemessen. Die Proben wurden in einem Glasversuch unter Anwendung von Standardverfahren behandelt, d. h. schnelles und langsames Mischen und Absetzen für 1 h, wonach die Messungen durchgeführt wurden. Die Ergebnisse zeigten, dass eine Erhöhung der Konzentration von Parrotia persica im Verhältnis zum Bentonit die Wirksamkeit der Behandlung verbesserte und dass die höchste Trübungsentfernung (> 96,8 %) durch Spülen der Mischungen mit Trinkwasser , Trocknen und dann erneuter Pulverisierung erreicht wurden. Potenziell toxische Metalle wurden zu mehr als 93,7 % entfernt. Die SEM-Mikrogramme weisen darauf hin, dass die Sorption ein physikalisch-chemisches Phänomen ist und dass der Ton und Parrotia persica eine zentrale Rolle spielen.
Resumen
Se realizaron pruebas en frascos sobre la eficacia del uso de varias mezclas de arcilla bentonita pulverizada y de la planta Parrotia persica para tratar el DAM procedente de una mina de la ciudad de Mogale (Sudáfrica) sin añadir alcalinidad. Se realizaron dos conjuntos de experimentos casi idénticos utilizando los mismos materiales después de haberlos lavado con agua destilada y potable, respectivamente, para eliminar las impurezas, y después de haberlos secado en un horno a 60 °C durante 24 h, tras lo cual se pulverizaron de nuevo. Se midieron el pH, la conductividad, el potencial de reducción de oxidación, la turbidez y las concentraciones de metales tóxicos antes y después de los experimentos. Las muestras se trataron en una prueba en jarra utilizando procedimientos estándar, es decir, mezcla rápida y lenta y sedimentación durante 1 h, tras lo cual se realizaron las mediciones. Los resultados mostraron que el aumento de la concentración de Parrotia persica en relación con la bentonita mejoraba la eficacia del tratamiento y que la mayor eliminación de turbidez (> 96,8%) se producía al lavar las mezclas con agua potable, secarlas y volver a pulverizarlas. Los metales potencialmente tóxicos se eliminaron en más de un 93,7%. Los microgramas de SEM indican que la sorción es un fenómeno físico-químico donde la arcilla y la Parrotia persica desempeñan un papel fundamental.
概括
摘 要:在不添加碱度的情况下,我们采用不同配比的粉状膨润土和波斯铁木植物粉对南非莫加尔市某矿山酸性矿井水的处理效果进行了实验。将相同的材料分成两组进行实验,两组样品分别使用蒸馏水和饮用水冲洗去除杂质,然后在60℃烘箱中干燥24 h,之后再次粉碎。实验前后分别测定了两组样品的pH、电导率、氧化还原电位、浊度和有毒金属浓度。样品在烧杯试验中使用标准程序进行处理,即快速和缓慢混合并沉降1小时之后进行测量。结果表明,相对于膨润土,增加波斯铁木粉的含量更能提高处理效果,并且使用饮用水冲洗、干燥、再粉碎后,混合物浊度的去除率最高达到>96.8%。其潜在有毒金属的去除率超过93.7%。SEM显微图表明,吸附是一种物理化学现象,粘土和波斯铁木在其中起着关键的作用。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Certain adsorbents, such as activated carbon (AC), are commonly used to treat wastewater (Hamza et al. 2017; Sadegh et al. 2016; Yu et al. 2014). Properties such as voluminous micropores, extensive surface area, extent of polarity, normal pore size distribution, highly reactive constituents, and appropriate surface chemistry characterise good adsorbents (Sagheb-Talebi et al. 2014). Studies have investigated mechanisms that could be used to produce AC of a better quality using low cost materials, such as wood, seeds, and dry leaves (Asadi-Samani et al. 2014; Barajas et al. 2016; Baruah et al. 2018; Wang et al. 2015). Several plants have been investigated and a Persian ironwood species, Parrotia persica emerged as an ideal choice because it is a lignocellulosic material with appropriate properties, such as high density and cellulose content, and is plentiful in moist forests (Sagheb-Talebi et al. 2014). P. persica is abundant in many underdeveloped countries but is not industrially harvested due to its low commercial value. A recent study conducted by Nowrouzi et al. (2017) measured CO2 uptake by AC prepared from pulverized P. persica, and the results revealed that its physical parameters, such as its high surface area, small pore diameter, and large micropore volume improved their CO2 uptake.
It is very important to select cost-effective reagents for the removal of turbid material, so in the present study, the dry seeds and stem of the P. persica plant were used as flocculants along with bentonite clay (BC) based on their ion exchange capacity, porous surface, high electronegativity, hydrolysing potential, etc. (Maree 2004; Ntwampe 2021a). The rate of physico-chemical reactions is largely influenced by the ionic strength of the suspension (Khan and Rao 2017) and calcium, magnesium, and potassium salts comprise up to 80% of the inorganic material present in the P. persica biomass. The sample also contained a variety of trace metals (Al, Ba, Co, Cr, Ga, Ag, Li, Sn, Sr, Ti, V, Zn, Fa, Mn, Pb, Mo, B, etc.), which contributed to the solution’s ionic strength and reactivity. These metals are likely derived from both anthropogenic and natural phenomena, i.e. industrial and domestic activities, volcanoes, wind dust, forest fires, and marine aerosols (Nagajyoti et al. 2010).
Among the advantages of natural flocculants/coagulants is the generation of low amounts of sludge, low alkalinity consumption during treatment, affordability, high efficiency, high biodegradability, and safety. Many indigenous plants can achieve effective flocculation in wastewater containing a variety of colloids over a broad range of dosage (Sanghi et al. 2006). In addition, they have been shown to be effective against and resistant to most water-borne pathogens (Kawo 2007).
This study was conducted to assess whether a mixture of BC and P. persica would effectively treat an AMD solution without AC and without pH adjustment. The novelty of this study was the simplicity of the technique and low equipment costs; the process can be operated by any well-trained technician. The reagents occur naturally and do not require sophisticated engineering processing. Water quality analysis, characterization, adsorptive capacity, and adsorption isotherm models were used to determine the efficiency of a mixture of unprocessed BC and P. persica for removing metals and turbidity from a sample of AMD.
Material and Methods
Acid Mine Drainage
The AMD sample was collected from metropolitan Mogale City (South Africa) in a 25 L plastic drum and kept in a lab refrigerator. Based on its low content of certain metals and semi-metals, we added 3.23, 2.5, 2.8, and 8.9 mg/L of As, Co, Zn, and Pb, respectively, to increase the concentration of potentially toxic contaminants. The pH, electrical conductivity (EC), and the turbidity of raw AMD sample were 2.25, 6.27 mS/cm, and 276 NTU respectively. The major elements present in the AMD sample were Cu, Zn, Co, Ni, Mn, Ti, Pb, Al, Fe, Se, Na, Mg, Ca, and K (Table 1).
The sample was placed in a refrigerator in a tightly closed plastic container to avoid air ingress. The parameters analysed in the AMD included the pH, turbidity, conductivity and oxidation/reduction potential (ORP) were 4.23, 5.86 mS/cm, 472 mV, and 293 NTU, respectively. The 200 mL of the AMD used during the experiment contained 11.7 g of solids.
Bentonite Clay
BC was obtained from the Yellowstar Bentonite mine (South Africa). The sample was pulverized to a very fine particle size straight from the supplier. 3 g of the BC was dissolved in 200 mL of distilled water and the pH, TSS, ORP, and conductivity were measured, showing 2.15, 13.5 mg/L, 445 mV, and 2.66 mS/cm, respectively.
The Parrotia persica Plant
Dry seeds and stem of the P. persica (supplemental Table S-1) plant were collected from Limpopo Province (South Africa). The sample was placed in an oven for drying at 40 °C for 24 h. The material was ground to fine powder (20 mesh) and the powder was kept in an air-tied plastic container at room temperature. 3 g of the P. persica was mixed with 200 mL of distilled water and the pH and EC were determined to be 6.8 and 5.7 mS/cm.
Flocculant Preparation and Jar Tests
The BC and P. persica were pulverised using a laboratory vibratory pulverizer (TF Laboratory Assay Equipment Factory, model IMZ-100, China), to a particle size of 180 μm. 25 g of each was then rinsed with distilled water to remove impurities using a 0.45 μm membrane filter to produce the materials for the flocculant tests using different BC: P. persica mass ratios. A trial test, test A, was conducted to determine the optimal flocculant dosage. The AMD samples were dosed with increasing (1.0–3.0 g) of BC while decreasing (3.0–1.0 g) the amount of P. persica to determine the best P. persica: BC ratio for the removal of pollutants in the AMD sample. The experiments involved the addition of 20–60 mL of the BC and P. persica mixture to the samples, which were rinsed with distilled water during preparation without processing in an oven; the results of the trial test are shown in supplemental Table S-2.
Two other flocculant tests (B and C) were prepared by pulverising the same reagents using mixtures containing 30, 25, 20, 15, and 10 g of BC and 10, 15, 20, 25, and 30 g of P. persica. In test A, the flocculant was prepared without processing in an oven, whereas the solid mixtures for tests B and C were inserted in an oven separately to dehydrate them at 60 °C for 24 h, after which the mixtures were cooled and then pulverised again to 180 μm before being used. In test B, the mixtures were placed in 500 mL of distilled water, while in test C, the distilled water was replaced with tap water. The pH, EC, and turbidity were 5.2, 6.4 mS/cm, and 109 NTU, respectively in test C, with insignificant deviations for tests A and B, i.e. 0.1–0.3, 0.2–0.4 mS/cm, and 4–6, respectively.
The jar test experiments were conducted using 200 mL of AMD poured into five 500 mL glass beakers. Different dosages were created by varying the BC: P. persica mass ratios, as mentioned above. The samples were mixed at 250 rpm for 2 min, then reduced to 100 rpm for 10 min, and allowed to settle for 1 h, after which the pH, conductivity, turbidity, and potentially toxic metals were measured.
Determination of the Oxidation/Reduction Potential
The ORP was calculated using an equation for measurements made using the standard hydrogen electrode (SHE) as the probe does not show the real figure (Wolkersdorfer 2008):
where E0 is reading of the SHE, E is the reading of the electrode (Mv), a and b are constants, and T is the temperature of the solution (°C)
Determination of the Coefficient of Linear Expansibility in Water
For each of the tests, 4 mL of the flocculants were poured into two separate 100 mL graduated cylinders and 40 mL of water was added and stirred at 50 rpm in a shaker for 24 h. The final volume of the flocculant was measured to determine the amount of hydration and swelling, which was calculated from the ratio between their initial and final volumes. The coefficient of linear expansibility (COLE) was then calculated by dividing the expansion volume of the clay by the original volume; a higher ratio is indicative of more expansion and hydration (Lucian 2008).
Performance Evaluation
A Mettler Toledo Seven multimeter (Germany) pH meter with an electrode filled was used. The equipment was calibrated with standard pH 4.0 and 7.0 buffer solutions before use. An EDT instrument FE 280 conductivity meter (Japan) was used and calibrated with a 0.1 KCl standard solution. A Merck Turbiquant 3000 T turbidimeter (Japan) was used to determine the turbidity. It was calibrated with 0.10, 10, 100, 1000, and 10,000 NTU standard solutions.
Inductively Coupled Plasma
An inductively coupled plasma (ICP) optical spectroscope (Perkin Elmer Optima DV 7000, USA) was used to measure the concentration of potentially toxic metals in the supernatant. Anticipating a high concentration, the ICP equipment was calibrated with a standard solution between 100 and 1000 mg/L.
Ion Chromatographic Method
A Dionex ICS 5000 ion chromatograph (Sunnyvale, USA) equipped with an IonPac AS12-AS anion column and suppressed conductivity detector was used to measure the chlorides and sulphates.
Scanning Electron Microscopic Analysis
A KYKY-EM3200 digital scanning electron microscope (SEM; model EM3200, China) was used.
Mathematical Modelling
The adsorption capacity was determined once the equilibrium concentration was obtained experimentally (Brdar et al. 2012) using the following equation:
where \({q}_{e}\) is the amount of pollutants adsorbed per unit mass of adsorbent (mg/g) at equilibrium; \({C}_{o}\) and C are ion concentrations in the solution initially and at equilibrium (mg/L); m is the amount of the pollutants (g) after contact with the adsorbent; V is the solution volume (L), and m is the mass of the dry adsorbent (g).
Determination of the adsorption capacity at a certain time interval was calculated using the following equation:
A pseudo-second order model was calculated using the following equation derived above:
where \({q}_{e}\) is the adsorbed amount of the turbid materials at equilibrium (mg/g), \({\mathrm{q}}_{\mathrm{t}}\) is the adsorbed amount of turbid materials at a certain time in (mg/g); and \({k}_{1}\mathrm{and }{k}_{2}\) are the rate constants for the first and second order adsorption kinetics, respectively. Non-linear regression methods were used to determine these rate constants.
Determination of the Adsorption Isotherm
Equilibrium adsorption is defined by an isotherm equation whereby the surface properties and attraction of the adsorbent are expressed by its parameters. The Freundlich and Langmuir isotherms are applied to determine the relationship between the amount of contaminant sorbed by the adsorbent and their equilibrium concentrations in aqueous solution (Ng et al. 2002). The Freundlich isotherm determines the amount of a solute adsorbed onto the surface of a solid and the amount of the solute which remained in the liquid. The Langmuir isotherm is used to determine whether a monolayer was shaped through reaction, existence corresponding positions, fixity of adsorbate, or absence of interactions between adsorbate-adsorbent (Günay et al. 2007). This isotherm also explains the situation when adsorption is limited to one molecular layer at or before a relative pressure of unity is reached. In contrast, the Freundlich isotherm is considered a multi-layer process where the amount of adsorbate per unit adsorbent increases gradually (Ng et al. 2002). The Langmuir isotherm reflects high amounts of sorption, while the Freundlich isotherm reflects infinite positions of variance between two models. The Freundlich isotherm is expressed as follows:
or in linearised form;
where: \({C}_{\mathrm{e}}\) (mg/L) is ions concentration in solution at equilibrium; \({q}_{\mathrm{e}}\) is the amount of pollutants adsorbed at equilibrium (mg/g); \({k}_{\mathrm{f}}\) is the Freundlich adsorption capacity parameter in (mg/g); and \(n\) is the Freundlich constant associated with the adsorption intensity and indicates the deviation of adsorption.
The Langmuir isotherm is expressed as follows:
where Ce (mg/L) is the metal concentration in solution at equilibrium, qe (mg/g) is the amount of metal adsorbed per unit mass of adsorbent, qm (mg/g) is the maximum adsorption capacity and b (L/g) is the Langmuir constant.
Results and Discussion
Figure 1 represents the pH, EC, and turbidity of the AMD treatment in the three tests, i.e. increasing amounts of unprocessed BC and decreasing amounts of P. persica and a combination of processed BC and P. persica rinsed with distilled water and tap water, respectively.
The pH of the samples in test A showed a decreasing trend with decreasing P. persica: BC mass ratios from 4.2 (raw AMD) to a range of 3.6–2.9; the pH of the samples in test B showed slightly lower values, also in a decreasing trend (3.5–2.3), whereas those from test C showed a slightly higher decreasing trend (4.1–2.6). Generally, increasing the P. persica: BC mass ratio increased the pH, whereas increasing the bentonite clay dosage decreased the pH of the samples due to the low pH of the bentonite clay (2.15). This reduction of AMD acidity was attributed to the precipitation of ferric hydroxide after the hydrolysis of Fe3+ ions, which is an indication of the efficiency of the flocculent. It is suggested that a fraction of the hydroxide ions that took part during hydrolysis existed due to the amphoteric nature of water molecules in the system (Ntwampe 2019).
The EC of the samples in tests A and B showed a decreasing trend with decreasing P. persica: BC mass ratios, from 5.9 mS/cm to ranges of 4.0–3.1 and 4.5–3.0 mS/cm, respectively. The EC of the samples in test C also showed a decreasing trend that was slightly higher than in tests A and B, i.e. a range of 5.8–3.4 mS/cm. In most cases, a decreasing EC trend is an indication of decreasing ionic strength, i.e. the precipitation of ionic charged contaminants or ionic compounds. Some of the ionic species in the samples cause a high EC, i.e. high ionic strength, enhancing the rate of physico-chemical reactions and removal of turbid material (Bergaya et al. 2006; Ntwampe 2019).
The residual turbidity in tests A and B showed high removal efficiencies from 293 NTU to the ranges of 22.8–34.4 and 19.4–29.8 NTU (an increasing trend with decreasing P. persica: BC mass ratios), respectively. The residual turbidity of the samples in test C were less than the residual turbidity of the samples in tests A and B, also with an increasing trend (14.9–24.4 NTU). This high removal efficiency, ranging from 91.6–94.9%, exceeded those in tests A and B (88.2–90.1 and 89.8–93.3%, respectively). The lower removal efficiency in test B is attributed to demineralization, which attenuated the ionic strength and reaction rates. The slightly lower efficiency in test A was attributed to a lack of compactness and coherence between the BC and P. persica, in contrast to the physical properties that resulted from the heating that took place in tests B and C.
Apart from that, it appears that the properties of the BC and P. persica also played a pivotal role in enhancing sorption; the physical properties mainly contributed to the sorption, whereas the chemical properties contributed during ionic exchange, cationic exchange capacity, and destabilization-hydrolysis. The chemical properties also contributed to the removal of metals by suppressing their solubility, i.e. the formation of insoluble metal hydroxide species. The pH range of the samples in tests C is conducive for the existence of all of the four species formed by hydrolysis of iron, namely Fe3+, Fe(OH)2+, Fe(OH)2+, and insoluble Fe(OH)3, as well as a minute fraction of the dimer, Fe2(OH)24+.These species have specific pH ranges at which they co-exist with some overlap. The pH values obtained from the results (Fig. 1) were in the range of 2.3–4.1, and that falls within the range of 1.0–6.5 that is dominated by Fe3+, Fe(OH)2+, Fe(OH)2+, and Fe(OH)3 species (Flynn 1984). This indicates that destabilization-hydrolysis, a precursor of turbidity removal, is not directly related to the pH during the coagulation-flocculation process but the physico-chemical properties of the reagent (the destabilization potential) and the colloidal suspension (double layer compression).
Apart from the physico-chemical mechanisms taking place during treatment, the properties of the natural reagents found in the P. persica and BC also played a prominent role in contaminant removal (Karmakar and Chandan 2019). The seeds of natural plants contain remarkable quantities of low molecular-weight proteins that are soluble in water. Crushing the seeds liberated both positively and negatively charged ions into the system, enhancing the reactivity. The proteins present in the seeds increased the concentration of the cations. The oppositely charged particles formed aggregates with their counterparts (neutralization), enhancing removal of the turbid material through “sweep flocculation” and interparticle bridging phenomena (Ntwampe 2016). The proteins may have also enhanced the reaction rate during coagulation-flocculation by acting as bonding agents for the negative charges, resulting in floc formation during destabilization-hydrolysis (Ntwampe 2019). The high settling velocity of these flocs resulted in contaminant removal (Fig. 1). It also suggests that the ability of the protein component of natural plants to attract suspended particulates and dissolved solids resulted in increasing mass and sedimentation, and also played a pivotal role during the coagulation-flocculation process. Treatment without pH adjustment could have played a major role due to the competition between the protons and the metal ions for the same functional groups, resulting in poor removal of turbid material, particularly potentially toxic metals, at a higher pH.
Supplemental Fig. S-1 shows the correlation between removal efficiencies and ORP. The samples in test A showed the lowest removal efficiency, from 472 mV (raw AMD) to a decreasing range of 71–83%, while the ORPs of the corresponding samples were higher than in tests B and C, indicating a lower rate of oxidation of the metals and/or other reactive material. The samples in test B showed higher removal efficiencies (a decreasing rate of 89–94%) and lower ORPs (334–362 mV) than those of test A, while the samples in test C showed the greatest removal efficiencies (95.8–98.3%) and the lowest corresponding ORPs (334–362 mV). The low ORPs of the test C samples is typically associated with higher metal oxidation rates, which can lead to the formation of insoluble and settleable metal hydroxide species (Ntwampe 2021b), resulting in floc formation and improved contaminant removal. Figure 2 illustrates the COLEs for the flocculants in water; these define the expansibility and swelling effect of the adsorbents.
According to the COLEs (Fig. 2), the higher ratio is indicative of greater expansion and hydration; the accuracy of this method was confirmed by the COLE values obtained. Test A showed the lowest COLE of 5.6, the COLE of test B was slightly higher (6.7), and test C had the highest (7.1), indicating that flocculant C exhibited the highest sorption capacity and the ability to swell on hydration. This was also expressed as high expansibility and dispersion in water, whereas the flocculents in test A exhibited limited swelling (Wang et al. 2020). The greater swelling tendency shown by the flocculents in test C was associated with their higher hydration energy. This enabled the hydration and desorption of the flocculents from interfacial regions following the addition of water.
The binding effect of the flocculants was investigated using equilibrium isotherms (supplemental Fig. S-2), since the residual turbid material in the treated effluent was determined by the adsorption capacity of the flocculant. The results showed that test C had a high flocculation ability due to its P. persica: BC ratio and pH, i.e. 3.0 and 4.1, respectively, as suggested by research conducted by Abdellsoui et al. (2019) while investigating the binding effect of clays to toxins. A coefficient regression greater than 0.8 and the randomness of the residuals were used to determine the best fit for the data (Freundlich model; Fig. 2A). Each point on the graph represents the values calculated for the turbid material bound to the flocculents in test C (mol/kg) and that remaining in the treated effluent (mol/L) for the corresponding dilutions. Although the expansibility of the sodium bentonite (SB) clay standard in water was larger than that of the calcium bentonite (CB) clay standard, its binding capacity was essentially lower (Fig. 2A). Since the pH for the flocculents in test C was 4.1, and that solution had a high concentration of protons, it is possible that the AMD sample may have released the protons, which neutralised the overall negative charge on the active surfaces of the interfacial region (Nandwani et al. 2019). In that regard, it is suggested that modifying the BC by mixing it with P. persica enhanced the performance of the flocculant as invoked by the results obtained on the modification of BC using tetramethylammonium chloride (Sponza et al. 2015). Physisorption and chemisorption cover many of the reactions occurring in water treatment; the former includes van der Waals forces of attraction, induced dipole interactions, dipole–dipole interaction, and hydrogen bonding, while chemisorption includes chemical reactions and sharing of electrons between the adsorbate and the adsorbent. Considerable turbidity removal suggests that large amounts were bound within the negatively charged interlayer of the flocculents in test C, with insignificant amounts bound on the edges and basal surfaces. A summary of the sorption parameters for turbid material onto the flocculant surfaces in test C is shown in Table 2.
The removal of potentially toxic metals from AMD typically involves pH adjustment to suppress their solubility through oxidation and enable the formation of insoluble metal hydroxide species. The results (Table 2) showed removal efficiencies in test C above 91.8%. On the other hand, some of the more common metals, such as aqueous calcium and magnesium (causing water hardness) reacted with anionic compounds (Ntwampe 2016). The residual trace elements can be removed by adsorption, interparticle bridging, intercalation, and entrapment by inter- and intra-fibrillary capillaries, which is attributed to the concentration gradient and diffusion (Nnaji 2012). In view of the chemical composition of the biomass, including the organic and inorganic components such as cellulose, hemicellulose, lignin, and extractives (hydrophobic and hydrophilic groups), the reaction rate and the removal efficiency would have been enhanced by the physical and chemical phenomena (Table 2). It is suggested that the adsorption of copper, zinc, cobalt, and nickel from the AMD sample in test C was improved by the alkali biomass. This is inferred by the increased acidic functional groups on the surface of the biomass during treatment, which increased their adsorption efficiencies (Zanin et al. 2017). The low oxygen shown by the ORP in test C (Table 2) shows that oxidation took place in the system where metals and/or other substances that behave as reducing agents formed cations, reacted with anions, and enhanced the removal of turbidity. On the other hand, oxygen-containing groups, such as hydroxyl, ether groups, and carboxyl originating from polyphenolic tannins, suberin, and flavonoids in the bark would have provided negatively charged surface sites, which then attracted positively charged metal ions (Fig. 1).
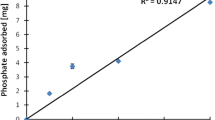
Figure 3 represents the adsorption capacity of the flocculents in test C obtained from the application of pseudo second-order reactions. The values were obtained by plotting the ratio of time and adsorption vs. contact time focusing on Cu, Zn, Ni, and As, and that was based on their potential toxicity. The correlation regression (R2) was determined using both the x and y values. After the experimental data excluded a pseudo-first model, the pseudo-second order parameters qe and k2 were calculated from the intercepts and the slope of t/qt and time.
The pseudo-second order model illustrates that the rate equation R2 values for Cu, Zn, Ni, and As adsorption in test C were close to unity, i.e. 0.993; 0.979; 0.98, and 0.992 respectively. The coefficient regression for As showed a value very close to unity compared to the others, although all exhibited high R2. The parameters qe and k2 obtained from the pseudo-second order were calculated from the intercepts, the slope of t/qt, and the time. Figure 4 shows the Freundlich model for Cu and Zn obtained in test C.
The adsorption of Cu and Zn fit the Freundlich model well, as shown by R2 values of 0.989 and 0.991, respectively (Fig. 4). This simply shows that the adsorption phenomenon was favourable in the conditions of test C, occurred spontaneously, and was an endothermic, physical process.
Figure 5 shows the Langmuir model for Cu and Zn for the results in test C. The R2 values of 0.867 and 0.891, respectively, indicates that the afore-mentioned conditions do not favour the Langmuir model, i.e. adsorption of the ions are not adsorbed in the surface monolayer, and the maximum adsorption did not occur when the surface was completely covered (Günay et al. 2007). Table 3 shows the parameters of test C for the Langmuir and Freundlich models. On the other hand, Table 4 illustrates the kinetic parameters of test C, applying a pseudo-second order.
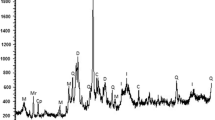
Figure 6 shows SEM micrographs of the AMD sludge from tests B and C. The dry sludge test B (Fig. 6A) showed large “sponge-like” flocs in the middle surrounded by smaller flocs. This is indicative of weaker bonds holding the flocs together and may have been caused by compromised forces of attraction due to low ionic strength because of the demineralization process. In contrast, the micrographs of the dry sludge from test C (Fig. 6B) exhibited dense flocs covering 90% of the slide. This showed the dominance of cohesive forces that produced a rigid structure showing adsorption features (Ayawei et al. 2017; Ntwampe 2021c). The bright colour shown by both micrographs shows the adsorption of metallic minerals on the porous surfaces of the adsorbents. The crystal morphology of both micrographs shows that the adsorption was a physico-chemical phenomenon.
Conclusions
This study determined the effect of mixing P. persica with BC to form a flocculant to treat AMD (supplemented with additional contaminants) without adding alkalinity. The removal efficiency of the turbid material was directly proportional to the concentration of P. persica, as shown by increasing the P. persica: BC mass ratio. The effectiveness of the P. persica is attributed to its high adsorption capacity and reactive affinity, which enables intermolecular forces of attraction associated with the adsorbate (flocculant) to concentrate or deposit some of the solute molecules on its surface, producing low residual levels of turbid materials in the treated AMD samples. The flocculents in test C exhibited an effective mass transfer process; the contaminants were transferred from the AMD sample to its surface and bound by physico-chemical interactions. This also indicates that the flocculant had a large surface area that enabled high adsorption capacity and surface reactivity. The removal of cations (e.g. Ca2+, Mg2+, Zn, Cu, Ni, As) is indicative of the effect of oxygen-containing functional. The removal of permanent hardness, i.e. the removal of Ca and Mg from 194 and 90 mg/L to 76 and 47 mg/L, respectively (Tables 1 and 3), also show removal by reactions between the cations and anionic compounds to form insoluble species (e.g. CaSO4, MgCO3, CaCO3). According to the COLE, the high ratio corresponds with increased expansion and hydration of the sample. The appropriateness of this method was invoked by the fact that the COLE values were lowest for the flocculents in test A, slightly higher for test B, and highest values in test C. This showed that rinsing the mixture (BC and P.persica) with potable water using minimum energy consumption (less milling time) was an effective method for turbidity removal provided an appropriate mass fraction between BC and P. persica is employed. The experimental data corresponded to the Freundlich model, which indicates that the process was thermodynamic in nature and occurred spontaneously, and that the adsorption was an endothermic and physical phenomenon. Further studies should be conducted to determine the feasibility of using a combination of BC and P. persica for AMD treatment in a pilot-scale test.
Data availability
The data is unavailable as such an information could not be found by the institution upon request citing misplacement during lockdown.
References
Asadi-Samani M, Bahmani M, Rafieian-Kopaei M (2014) The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pac J Trop Med 7(l1):S22–S28
Ayawei N, Ebeleg AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem. https://doi.org/10.1155/2017/3039817
Barajas J, Latayan J, Pagsuyoin S, Bacani F, Santos J, Tan R, Orbecido A, Razon L, Almendrala M (2016) Water disinfection using Moringa protein adsorbed on rice husk ash. Proc DLSU Res Congress De La Salle Univ Manila Philippines 4:16–19
Baruah J, Nath BK, Sharma R, Kumar S, Deka FC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res. https://doi.org/10.3389/fenrg.2018.00141
Bergaya F, Theng BKG, Lagaly G (2006) Handbook of clay science. Elsevier Ltd 1:1–18
Brdar M, Šćiban M, Takači A, Došenović T (2012) Comparison of two and three parameters adsorption isotherm for Cr(VI) onto Kraft lignin. Chem Eng J 183:108–111
Flynn CM (1984) Hydrolysis of Inorganic Iron(III). J Am Chem Soc 84:31–41
Günay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146(1–2):362–371
Hamza L, Khefile A, Belhamdi B, Trari M (2017) Valorization of coffee grounds into activated carbon using physic—chemical activation by KOH/CO2. J Environ Chem Eng 5(5):5061–5066
Karmakar GP, Chandan K (2019) Flocculation behaviour of bentonite clay-water systems. Adv Mater Process Technol 5(2):303–316. https://doi.org/10.1080/2374068X.2019.1583935
Kawo AH (2007) Water purification potentials and in vivo toxicity evaluation of the aqueous and petroleum ether extracts of Calotropis procera, latex and Moringa oleifera Lam seed powder. PhD thesis, Microbiology Unit, Dept of Biological Sciences, Bayero, Univ Kano, Nigeria
Khan U, Rao RAK (2017) A high activity adsorbent of chemically modified Cucurbita moschata (a novel adsorbent) for the removal of Cu(II) and Ni(II) from aqueous solution: synthesis, characterization and metal removal efficiency. Proc Safe Environ Prot 107:238–258. https://doi.org/10.1016/j.psep.2017.02.008
Lucian C (2008) Geotechnical Aspects of Buildings on Expansive Soils in Kibaha, Tanzania. PhD thesis, Royal Institute of Technology, Stockholm
Maree JP (2004) Treatment of industrial effluent for neutralization and sulphate removal. PhD thesis, North West Univ, South Africa
Murkovic M (2002) The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J Biochem Biophys Meth 53:45–49
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216. https://doi.org/10.1007/s10311-010-0297-8
Nandwani SK, Chakraborty M, Gupta S (2019) Adsorption of surface active ionic liquids on different rock types under high salinity conditions. Sci Rep. https://doi.org/10.1038/s41598-019-51318-21
Ng OC, Losso JN, Marshall WE, Rao RM (2002) Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin-water system. Bioresour Technol 85(2):131–135
Nnaji PC (2012) An investigation of the performance of various coagulants/flocculants in removing the turbidity of coal washery effluents. M Eng Thesis, Federal Univ of Technology, Owerri
Nowrouzi M, Younesi H, Bahramifar N (2017) High efficient carbon dioxide capture onto as-synthesized activated carbon by chemical activation of Persian ironwood biomass and the economic pre-feasibility study for scale-up. J Clean Prod 168(17):499–509
Ntwampe IO (2016) Comparison of chemical reactivity between inorganic and synthetic polymers in the treatment of AMD. M Eng thesis, North-West Univ, South Africa
Ntwampe OI (2019) The effect of saw dust in a flocculent with bentonite clay and FeSO4 in AMD treatment without addition of a neutralizer. Water Pract Technol 14(3):633–644. https://doi.org/10.2166/wpt.2019.051
Ntwampe IO (2021a) Treatment of AMD using a combination of saw dust, bentonite clay and phosphate in the removal of toxic metals. Water Pract Technol 16(2):541–556. https://doi.org/10.2166/wpt.2021.014
Ntwampe IO (2021b) The removal of turbidity and toxic metals in the AMD using a combination of saw dust, bentonite clay and synthetic CaMg·2(OH)2. Water Pract Technol 16(4):1234–1247
Sadegh H, Ghoshekandi RS, Masjedi A, Mahmoodi Z, Kazemi M (2016) A review on carbon nanotubes adsorbents for the removal of pollutants from aqueous solutions. Int J Nanomedicine 7(2):109–120
Sagheb-Talebi K, Sajedi T, Pourhashemi M (2014) Forests of Iran: a treasure from the past, a hope for the future, 1st edn. Springer, Netherlands
Sanghi R, Bhattacharya B, Dixit A, Singh V (2006) Ipomoea dasysperma seed gum: an effective natural coagulant for the decolorization of textile dye solutions. J Environ Manag 81(1):36–41
Sato K, Barast G, Razakamanantsoa AR, Irini DM, Katsumi T, Levacher D (2016) Comparison of prehydration and polymer adding effects on Na activated Ca-bentonite by free swell index test. Appl Clay Sci. https://doi.org/10.1016/j.clay.2016.10.009
Sponza A, Fermandez N, Navarro A (2015) Comparative sorption of methylene blue onto hydrophobic clays. Environments 2(3):388–398
Wang K, Zhang J, Shanks BH, Brown RC (2015) The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation. Appl Energy 148:115–120. https://doi.org/10.1016/j.apenergy.2015.03.034
Wang M, Hearon SE, Phillips TD (2020) A high capacity bentonite clay for the sorption of aflatoxins. Food Addit Contam Part A 37(2):332–341
Wolkersdorfer C (2008) Water management at abandoned flooded underground mines. Springer, Berlin
Yu JG, Zhao XH, Yu LY, Jiao FP, Jiang JH, Chen X (2014) Removal, recovery and enrichment of metals from aqueous solutions using carbon nanotubes. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-013-2818-y
Zanin E, Scapinello J, Oliveira M, Rambo CL, Franscescon F, Freitas L, Mello JMM, Fiori MA, Oliveira JV, Dal J (2017) Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf Environ Prot 105:194–200
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
10230_2023_936_MOESM1_ESM.docx
Table S-1: Composition of natural plant (Murkovic 2002). Table S-2: pH, EC and RT of the trial experiment (DOCX 16 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ntwampe, I.O. Adsorption Efficiency of Bentonite Clay and Parrotia persica During Acid Mine Drainage Treatment for the Removal of Turbid Material. Mine Water Environ 42, 348–357 (2023). https://doi.org/10.1007/s10230-023-00936-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-023-00936-4