Abstract
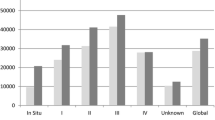
New and emerging advances in colorectal cancer (CRC) treatment combined with limited healthcare resources highlight the need for detailed decision-analytic models to evaluate costs, survival and quality-adjusted life years. The objectives of this article were to estimate the expected lifetime treatment cost of CRC for an average 70-year-old patient and to test the applicability and flexibility of a model in predicting survival and costs of changing treatment scenarios. The analyses were based on a validated semi-Markov model using data from a Norwegian observational study (2049 CRC patients) to estimate transition probabilities and the proportion resected. In addition, inputs from the Norwegian Patient Registry, guidelines, literature, and expert opinions were used to estimate resource use. We found that the expected lifetime treatment cost for a 70-year-old CRC patient was €47,300 (CRC stage I €26,630, II €38,130, III €56,800, and IV €69,890). Altered use of palliative chemotherapy would increase the costs by up to 29%. A 5% point reduction in recurrence rate for stages I–III would reduce the costs by 5.3% and increase overall survival by 8.2 months. Given the Norwegian willingness to pay threshold per QALY gained, society’s willingness to pay for interventions that could result in such a reduction was on average €28,540 per CRC patient. The life years gained by CRC treatment were 6.05 years. The overall CRC treatment costs appear to be low compared to the health gain, and the use of palliative chemotherapy can have a major impact on cost. The model was found to be flexible and applicable for estimating the cost and survival of several CRC treatment scenarios.

Reproduced from [5] with kind permission from Sage publishers

Reproduced from [5] with kind permission from Sage publishers
Similar content being viewed by others
Availability of data and material
The Markov model and the data used are presented in a separate article (1). For modelling the Markov model, we used Excel 2016, and for the PSA we used @risk 7.5 for Excel from Palisade.
Abbreviations
- 5-FU/FA:
-
Nordic FLv = 5-fluorouracil/folinic acid
- CI:
-
Confidence interval
- COI:
-
Cost-of-illness
- CRC:
-
Colorectal cancer
- CrI:
-
Credible interval
- EGFR-inh:
-
Epidermal growth factor receptor inhibitors (cetuximab/panitumumab)
- FLIRI:
-
A combination of irinotecan and 5-fluorouracil/folinic acid
- FLOX:
-
A combination of oxaliplatin and 5-FU/FA
- FOBTs:
-
Faecal occult blood tests
- HRQoL:
-
Health-related quality of life
- LYs:
-
Life years
- NPR:
-
National Patient Registry
- OUS:
-
Oslo University Hospital
- PSA:
-
Probabilistic sensitivity analysis
- PS:
-
Patient performance status
- QALY:
-
Quality-adjusted life years
- WTP:
-
Willingness to pay
References
Jemal, A., Center, M.M., DeSantis, C., Ward, E.M.: Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 19(8), 1893–1907 (2010)
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J.W.W., Comber, H., et al.: Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49(6), 1374–1403 (2013)
Tarricone, R.: Cost-of-illness analysis. Health Policy 77(1), 51–63 (2006)
Sullivan, S.D., Mauskopf, J.A., Augustovski, F., Jaime Caro, J., Lee, K.M., Minchin, M., et al.: budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 17(1), 5–14 (2014)
Joranger, P., Nesbakken, A., Hoff, G., Sorbye, H., Oshaug, A., Aas, E.: Modeling and validating the cost and clinical pathway of colorectal cancer. Med. Decis. Mak. 35(2), 255–265 (2014). https://doi.org/10.1177/0272989X14544749
Meltzer, D.: Accounting for future costs in medical cost-effectiveness analysis. J. Health Econ. 16(1), 33–64 (1997)
Drummond, M., Sculpher, M., Torrance, G., O’Brien, B., Stoddart, G.: Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University Press, Oxford (2005)
Sjo, O.H., Lunde, O.C., Nygaard, K., Sandvik, L., Nesbakken, A.: Tumour location is a prognostic factor for survival in colonic cancer patients. Colorectal Dis. 10(1), 33–40 (2008)
Nesbakken, A., Nygaard, K., Westerheim, O., Mala, T., Lunde, O.C.: Local recurrence after mesorectal excision for rectal cancer. Eur. J. Surg. Oncol. 28(2), 126–134 (2002)
Aas E (2009) Cost-effectiveness of screening for colorectal cancer with once-only flexible sigmoidoscopy and faecal occult blood test. In: Oslo University, Health Economics Research Programme
Yabroff, K.R., Lawrence, W.F., Clauser, S., Davis, W.W., Brown, M.L.: Burden of illness in cancer survivors: findings from a population-based national sample. J. Natl. Cancer Inst. 96(17), 1322–1330 (2004). https://doi.org/10.1093/jnci/djh255
Saarni, S.I., Härkänen, T., Sintonen, H., Suvisaari, J., Koskinen, S., Aromaa, A., et al.: The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual. Life Res. 15(8), 1403–1414 (2006)
Do, Health: Economic evaluation of health intervention: a guide. The Norwegian Directorate of Health, Oslo (2012)
Mo, Finance: Guid for cost-benefit analysis. The Treasury Department, Oslo (2005)
Finance Mo: Principles and requirements for the preparation of socio-economic analyzes. Ministry of Finance, Oslo (2014)
Razenberg, L.G.E.M., Creemers, G.-J., Beerepoot, L.V., Vos, A.H., van de Wouw, A.J., Maas, H.A.A.M., et al.: Age-related systemic treatment and survival of patients with metachronous metastases from colorectal cancer. Acta Oncol. 55(12), 1443–1449 (2016)
Sorbye, H., Pfeiffer, P., Cavalli-Björkman, N., Qvortrup, C., Holsen, M.H., Wentzel-Larsen, T., et al.: Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 115(20), 4679–4687 (2009)
Scholefield, J.H., Moss, S.M., Mangham, C.M., Whynes, D.K., Hardcastle, J.D.: Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 61(7), 1036–1040 (2012)
RCPH (2007) Screening for colorectal cancer in Vejle and Copenhagen County: Research Centre for Prevention and Health (RCPH)
Tappenden, P., Chilcott, J., Eggington, S., Sakai, H., Karnon, J., Patnick, J.: Option appraisal of population-based colorectal cancer screening programmes in England. Gut 56(5), 677–684 (2007)
Frazier, A.L., Colditz, G.A., Fuchs, C.S., Kuntz, K.M.: Cost-effectiveness of screening for colorectal cancer in the general population. JAMA 284(15), 1954–1961 (2000)
Tappenden, P., Eggington, S., Nixon, R., Chilcott, J., Sakai, H., Karnon, J.: Colorectal cancer screening options appraisal: cost-effectiveness, cost-utility and resource impact of alternative screening options for colorectal cancer. University of Sheffild, Sheffild (2004)
Ladabaum, U., Phillips, K.A.: Colorectal cancer screening: differential costs for younger versus older Americans. Am. J. Prev. Med. 30(5), 378–384 (2006)
Brown, M.L., Riley, G.F., Potosky, A.L., Etzioni, R.D.: Obtaining long-term disease specific costs of care: application to medicare enrollees diagnosed with colorectal cancer. Med. Care 37(12), 1249–1259 (1999)
Yabroff, K.R., Borowski, L., Lipscomb, J.: Economic studies in colorectal cancer: challenges in measuring and comparing costs. JNCI Monogr. 2013(46), 62–78 (2013)
Tilson, L., Sharp, L., Usher, C., Walsh, C., Whyte, S., O’Ceilleachair, A., et al.: Cost of care for colorectal cancer in Ireland: a health care payer perspective. Eur. J. Health Econ. 13(4), 511–524 (2012)
Van Cutsem, E., Cervantes, A., Adam, R., Sobrero, A., Van Krieken, J.H., et al.: ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27(8), 1386–1422 (2016). https://doi.org/10.1093/annonc/mdw235
Tejpar, S., Stintzing, S., Ciardiello, F., et al.: Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the crystal and fire-3 trials. JAMA Oncol. 3(2), 194–201 (2017)
Norderhaug, I.T.H.: Pasientvolum og kvalitet ved koloncancerkirurgi. Nasjonalt kunnskapssenter for helsetjenesten, Oslo (2009)
Meyerhardt, J.A., Giovannucci, E.L., Holmes, M.D., Chan, A.T., Chan, J.A., Colditz, G.A., et al.: Physical activity and survival after colorectal cancer diagnosis. J. Clin. Oncol. 24(22), 3527–3534 (2006)
Meyerhardt, J.A., Heseltine, D., Niedzwiecki, D., Hollis, D., Saltz, L.B., Mayer, R.J., et al.: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J. Clin. Oncol. 24(22), 3535–3541 (2006)
Meyerhardt, J.A., Giovannucci, E.L., Ogino, S., Kirkner, G.J., Chan, A.T., Willett, W., et al.: Physical activity and male colorectal cancer survival. Arch. Intern. Med. 169(22), 2102–2108 (2009)
Lynch, B.M., Cerin, E., Owen, N., Aitken, J.F.: Associations of leisure-time physical activity with quality of life in a large, population-based sample of colorectal cancer survivors. Cancer Causes Control 18(7), 735–742 (2007)
Haydon, A.M., MacInnis, R.J., English, D.R., Giles, G.G.: Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55(1), 62–67 (2006)
Meyerhardt, J.A., Niedzwiecki, D., Hollis, D., Saltz, L.B., Hu, F.B., Mayer, R.J., et al.: Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298(7), 754–764 (2007)
Huxley, R.R., Ansary-Moghaddam, A., Clifton, P., Czernichow, S., Parr, C.L., Woodward, M.: The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int. J. Cancer 125(1), 171–180 (2009)
Rock, C.L., Doyle, C., Demark-Wahnefried, W., Meyerhardt, J., Courneya, K.S., Schwartz, A.L., et al.: Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 62(4), 242–274 (2012)
Ravasco, P., Monteiro-Grillo, I., Camilo, M.: Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 96(6), 1346–1353 (2012)
Hamers, P., Bos, A.C.R.K., May, A.M., Punt, C.J.A., Koopman, M., Vink, G.R.: Recent changes in overall survival of real-life stage IV colorectal cancer patients. J. Clin. Oncol. 37(15_suppl), 3522 (2019)
Acknowledgements
We acknowledge the Department of Health, Nutrition and Management (HEL), The Faculty of Health Sciences, and Oslo and Akershus University College of Applied Sciences for funding Paal Joranger’s doctorate.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed to the work, are aware of, and agree to the submission. Any other person or body with an interest in the manuscript, such as our funders and employers, are also aware and agreed on the submission. Paal Joranger, Eline Aas, and Arne Oshaug declare no support from any organisation for the submitted work, no financial relationships in the previous 3 years with any organisations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work. In 2017, Geir Hoff received payment from Amgen Norway for giving a lecture at a medical conference. Halfdan Sorbye received Grants and personal fees from Merck, Roche, and Amgen and personal fees from Sanofi during the study. Arild Nesbakken received funding from Helse Sør-Øst for the clinical studies on colorectal cancer (OUS-Aker series). He is a member of a research group in OUS, which has patents on one diagnostic and two prognostic genetic tests for colorectal cancer. He received payment from Amgen Norway for giving a lecture at a medical conference 2018. Financial support for this study was provided entirely by the authors’ employers, which are listed above. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the article. The authors declare that they have no competing interests.
Ethics approval and consent to participate
The observational study from 1993 to 2010, including 2049 patients diagnosed with CRC at Oslo University Hospital, was approved by the Regional Ethics Committee (Norway) for Medical Research (REK approval 1.2005.1629). The study with data collected from the National Patient Register (NPR) was approved by the Regional Ethics Committee (Norway). The reference number is S-02113 (2013/83).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joranger, P., Nesbakken, A., Sorbye, H. et al. Survival and costs of colorectal cancer treatment and effects of changing treatment strategies: a model approach. Eur J Health Econ 21, 321–334 (2020). https://doi.org/10.1007/s10198-019-01130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01130-6