Abstract
Background
External reference pricing (ERP) is widely used to regulate drug prices. Although the literature has largely focused on the impact of ERP on a number of policy endpoints and its impact from a geographical perspective, a comparative study drawing on evidence from different settings does not exist to date.
Methods
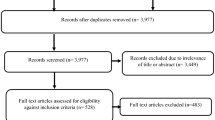
A systematic literature review was conducted on pre-defined endpoints on the impact of ERP across countries, such as price stability, price convergence and launch delays. Expert consultation was undertaken to analyse whether or not the international implications of ERP are considered during its design.
Results
46 studies were included in the analysis. Across countries, ERP may cause launch delays, price instability and lead to price convergence. However, these effects cannot be solely attributed to ERP, as there may be other factors at play, such as the size and the GDP of a country and other regulations in place, which can trigger these effects or reduce their effect. Nevertheless, the nature of ERP facilitates these unintended consequences and directly links them to it. Despite these cross-country implications being well known to decision-makers, they are not necessarily considered during the design of ERP.
Conclusions
As the effects of ERP as a stand alone policy are very difficult to isolate in the presence of other regulatory measures implemented within countries and the presence of other extrinsic factors across countries, our findings are inconclusive. Still, there is an unquestionable unmet need related to the design of ERP systems to attain a positive impact internationally.

Source: The authors
Similar content being viewed by others
References
World Health Organization: WHO Guideline on Country Pharmaceutical Pricing Policies. World Health Organization, Geneva (2013)
Espin, J., Rovira, J., Ewen, M., Laing, R.: Mapping external reference pricing practices for medicines. Health Action International and the Andalusian School of Public Health (2014)
Houy, N., Jelovac, I.: Drug approval decision times, international reference pricing and strategic launches of new drugs. International Reference Pricing and Strategic Launches of New Drugs (2014)
Leopold, C., Mantel-Teeuwisse, A.K., Seyfang, L., Vogler, S., de Joncheere, K., Laing, R.O., Leufkens, H.: Impact of external price referencing on medicine prices—a price comparison among 14 European countries. South. Med. Rev. 5(2), 34–41 (2012)
Leopold, C., Vogler, S., Mantel-Teeuwisse, A.K., de Joncheere, K., Leufkens, H.G.M., Laing, R.: Differences in external price referencing in Europe—a descriptive overview. Health Policy 104(1), 50–60 (2012). https://doi.org/10.1016/j.healthpol.2011.09.008
World Health Organization: Regional Office for Europe: Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research (2015). http://www.euro.who.int/__data/assets/pdf_file/0008/306179/Access-new-medicines-TR-PIO-collaboration-research.pdf?ua=1
Kanavos, P., Nicod, E., Espin, J., Van Den Aardweg, S.: Short-and long-term effects of value-based pricing vs. external price referencing. Eminet. LSE (2010)
OECD: Pharmaceutical pricing policies in a global market. Organisation for economic co-operation and development (OECD), Paris (2008)
Toumi, M.: External reference pricing of medicinal products: simulation-based considerations for cross-country coordination. European Commission, Brussels. https://ec.europa.eu/health/sites/health/files/systems_performance_assessment/docs/erp_reimbursement_medicinal_products_en.pdf (2014). Accessed 25 Sept 2015
European Commission: Study on enhanced cross-country coordination in the area of pharmaceutical pricing. https://ec.europa.eu/health/sites/health/files/systems_performance_assessment/docs/pharmaproductpricing_frep_en.pdf (2015). Accessed 8 June 2016
Danzon, P.M., Wang, Y.R., Wang, L.: The impact of price regulation on the launch delay of new drugs—evidence from twenty-five major markets in the 1990s. Health Econ. 14(3), 269–292 (2005). https://doi.org/10.1002/hec.931
Europe Economics: External Reference Pricing. http://www.europe-economics.com/publications/external_reference_pricing_-_final_report.pdf (2013). Accessed 25 Sept 2015
Espin, J., Rovira, J.: Analysis of differences and commonalities in pricing and reimbursement systems in Europe. Brussels: DG Enterprise and Industry of the European Commission 100 (2007)
Kaló, Z., Akhras, K.S., Voko, Z., Kanavos, P.: The implications of external price referencing of pharmaceuticals in Middle East countries. Expert. Rev. Pharmacoecon. Outcomes Res. (2015). https://doi.org/10.1586/14737167.2016.1136792
Kanavos, P., Gill, J., Efthymiadou, A., Fontrier, A.-M.: Does external reference pricing deliver what it promises? Evidence on its impact: in review (2019)
Gill, J., Kanavos, P., Kyriopoulos, D., Fontrier, A-M.: Variation in external reference pricing implementation—does it matter for public policy? in review (2019)
Kanavos, P., Fontrier, A.M., Gill, J., Efthymiadou, O., Boekstein, N. The impact of external reference pricing within and across countries. LSE (2017). https://doi.org/10.21953/lse.m0bluqcv10g6
Centre for Reviews and Dissemination (CRD): Systematic Reviews—CRD’s guidance for undertaking reviews in health care, University of York (2009)
Kanavos, P., Ross-Degnan, D., Fortess, E., Abelson, J., Soumerai, S.: Chapter 5: Measuring, monitoring and evaluating policy outcomes in the pharmaceutical sector. Eur. Observ. Health Syst. Policies Ser. 1, 97–113 (2004)
Rémuzat, C., Urbinati, D., Mzoughi, O., El Hammi, E., Belgaied, W., Toumi, M.: Overview of external reference pricing systems in Europe. J Mark Access Health Policy (2015). https://doi.org/10.3402/jmahp.v3.27675
Håkonsen, H., Horn, A.M., Toverud, E.L.: Price control as a strategy for pharmaceutical cost containment—what has been achieved in Norway in the period 1994–2004? Health Policy 90(2–3), 277–285 (2009). https://doi.org/10.1016/j.healthpol.2008.09.018
Leopold, C., Mantel-Teeuwisse, A., Vogler, S., Joncheere, K., Laing, R., Leufkens, H.: Is Europe still heading to a common price level for on-patent medicines? An exploratory study among 15 western European countries. Health Policy 112(3), 209–216 (2013). https://doi.org/10.1016/j.healthpol.2013.08.012
Ruggeri, K., Nolte, E.: Pharmaceutical pricing: the use of external reference pricing. Rand. Health Q. 3, 6 (2013)
Business Monitor International: Italy Pharmaceuticals and Healthcare Report—Q3 2009, pp. 1–67. Business Monitor International, London (2009)
Business Monitor International: Italy Pharmaceuticals and Healthcare Report—Q4 2009, pp. 1–75. Business Monitor International, London (2009)
Business Monitor International: Italy Pharmaceuticals and Healthcare Report—Q3 2010, pp. 1–74. Business Monitor International, London (2010)
Business Monitor International: Italy Pharmaceuticals and Healthcare Report—Q2 2010, pp. 1–78. Business Monitor International, London (2010)
Business Monitor International: Italy Pharmaceuticals and Healthcare Report—Q1 2010, pp. 1–74. Business Monitor International, London (2010)
Lu, C.Y.: The pharmaceutical policy environment and pharmaceutical pricing policies. In: Pharmaceutical Prices in the 21st Century, pp. 403–411 (2015)
Kanavos, P.G., Vandoros, S.: Determinants of branded prescription medicine prices in OECD countries. Health Econ. Policy Law 6(3), 337–367 (2011). https://doi.org/10.1017/s1744133111000090
Business Monitor International: Switzerland Pharmaceuticals and Healthcare Report—Q3 2010, pp. 1–77. Business Monitor International, London (2010)
Business Monitor International: Germany Pharmaceuticals and Healthcare Report—Q1 2011, pp. 1–97. Business Monitor International, London (2011)
Business Monitor International: Germany Pharmaceuticals and Healthcare Report—Q2 2011, pp. 1–102. Business Monitor International, London (2011)
Business Monitor International: Switzerland Pharmaceuticals and Healthcare Report—Q4 2011, pp. 1–88. Business Monitor International, London (2011)
Business Monitor International: Switzerland Pharmaceuticals and Healthcare Report—Q1 2012, pp. 1–86. Business Monitor International, London (2012)
Houy, N., Jelovac, I.: Drug launch timing and international reference pricing. Health Econ. 24(8), 978–989 (2013)
Danzon, P.M., Towse, A.: Differential pricing for pharmaceuticals: reconciling access, R&D and patents. Int. J. Health Care Financ. Econ. 3(3), 183–205 (2003)
Vogler, S., Habimana, K.: Study of the Policy Mix for the Reimbursement of Medicinal Products. Proposal for a Best Practice-Based Approach Based on Stakeholder Assessment. European Commission, Brussels (2014)
Vogler, S., Vitry, A., Babar, Z.U.: Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol. 17(1), 39–47 (2016). https://doi.org/10.1016/s1470-2045(15)00449-0
Vogler, S., Zimmermann, N., Ferrario, A., Wirtz, V.J., Babar, Z.U.: Challenges and opportunities for pharmaceutical pricing and reimbursement policies. J. Pharm. Policy Pract. 8 (Suppl 1 Abstracts from the 3rd International PPRI Conference), E1 (2015). https://doi.org/10.1186/2052-3211-8-s1-e1
Kaló, Z., Docteur, E., Moïse, P.: Pharmaceutical Pricing and Reimbursement Policies in Slovakia. Federal Reserve Bank of St Louis, St Louis (2008)
Business Monitor International: Taiwan Pharmaceuticals and Healthcare Report—Q3 2012, pp. 1–100. Business Monitor International, London (2012)
Business Monitor International: Taiwan Pharmaceuticals and Healthcare Report—Q3 2014, pp. 1–137. Business Monitor International, London (2014)
Business Monitor International: Turkey Pharmaceuticals and Healthcare Report—Q4 2015, pp. 1–127. Business Monitor International, London (2015)
OECD: Competition Issues in the Distribution of Pharmaceuticals: Contribution from Romania—Session III. Global Forum on Competition (2014). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=DAF/COMP/GF/WD(2014)30&docLanguage=En. Accessed 3 Feb 2014
OECD: Competition Issues in the distribution of pharmaceuticals: Background Note by the Secretariat—Session III—Global Forum on Competition (2014). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=DAF/COMP/GF(2014)3&docLanguage=En. Accessed 3 Feb 2014
OECD: Competition Issues in the Distribution of Pharmaceuticals: Contribution from the European Union—Session III—Global Forum on Competition (2014). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=DAF/COMP/GF/WD(2014)32&docLanguage=En. Accessed 30 Jan 2014
OECD: Competition Issues in the Distribution of Pharmaceuticals: Summary of Discussion. Global Forum on Competition (2014). http://www.fne.gob.cl/wp-content/uploads/2014/09/2014-Competition-Issues-in-the-Distribution-of-Pharmaceuticals-289-KB.pdf. Accessed 23 July 2014
OECD: Competition Issues in the Distribution of Pharmaceuticals: Contribution from BIAC—Session III—Global Forum on Competition (2014). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=DAF/COMP/GF/WD(2014)57&docLanguage=En. Accessed 24 Feb 2014
Rudisill, C., Vandoros, S., Antoun, J.G.: Pharmaceutical policy Reform in the Russian Federation. J. Health Polit. Policy Law 39(3), 691–705, 615 (2014). https://doi.org/10.1215/03616878-2682659
Mossialos, E., Brogan, D., Walley, T.: Pharmaceutical pricing in Europe: weighing up the options. Int. Soc. Secur. Rev. 59(3), 3 (2006). https://doi.org/10.1111/j.1468-246X.2006.00245.x
Barros, P.P.: Pharmaceutical Policies in European Countries. Pharmaceutical Markets and Insurance Worldwide (Advances in Health Economics and Health Services Research 22 (2010). https://doi.org/10.1108/s0731-2199(2010)0000022004
LSE Health/Medical Technology Research Group (MTRG): Survey of Key Experts on the Regulation of Medical Technology. http://www.lse.ac.uk/lse-health/research/mtrg (2017)
Akehurst, R., Abadie, E., Renaudin, N., Sarkozy, F.: Variation in health technology assessment and reimbursement processes in Europe. Value Health 20, 67–76 (2017)
Jaksa, A., Pontynen, A., Bastian, A.: Exploring the time delay between regulatory approval and health technology assessments (HTAs) of oncology therapies in France, Germany, England, Scotland, Canada, and Australia. J. Clin. Oncol. 35(15_suppl), 6545 (2017). https://doi.org/10.1200/jco.2017.35.15_suppl.6545
Jaksa, A., Pontynen, A., Bastian, A.: An exploration of how a countries’ HTA process can affect patient access. J. Clin. Oncol. 35(15), e18052 (2017). https://doi.org/10.1200/jco.2017.35.15_suppl.e18052
Clement, F.M., Harris A, Li, J.J., Yong, K., Yong K, Lee, K.M., Manns, B.J.: Using Effectiveness and Cost-Effectiveness to Make Drug Coverage Decisions: A Comparison of Britain, Australia, and Canada (1538–3598 (Electronic))
Nicod, E., Kanavos, P.: Commonalities and differences in HTA outcomes: a comparative analysis of five countries and implications for coverage decisions (1872–6054 (Electronic))
Sullivan, S.D., Kanavos, P., Kaló, Z.: Best Practice Principles for External Price Referencing of Medicines; London School of Economics (2019)
Acknowledgements
We are grateful to Mackenzie Mills, Nicola Boekstein, Olina Efythimiadou, Erica Visintin, David Taylor and two anonymous referees for valuable comments and suggestions. We would also like to thank Ibrahim Abbadi, Jakub Adamski, Daoud Al-Badriyeh, Diāna Arāja, Anya Bazhenova, Tatyana Benisheva, Irina Cleemput, Nevine Elnahass, Jaime Espin, Zoltán Kalo, Chara Kani, Gabor Lengyel, Vlad Mixich, Anban Pillay, Valentina Rupel, Marisa Santos, Tomas Tesar, Joice Valentim and Kärt Veliste for their personal input. This paper was undertaken in the context of the LSE Health-Medical Technology Research Group Pharmaceutical Regulation programme. All outstanding errors remain our own.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fontrier, AM., Gill, J. & Kanavos, P. International impact of external reference pricing: should national policy-makers care?. Eur J Health Econ 20, 1147–1164 (2019). https://doi.org/10.1007/s10198-019-01083-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01083-w
Keywords
- External reference pricing
- Pharmaceutical pricing
- Pharmaceutical policy
- Systematic review
- Regulation of pharmaceuticals
- Expert consultation