Abstract
The objective of the present study was to describe a new model of the cost-effectiveness of treatment of generalized anxiety disorder (GAD) and its application to a comparison of pregabalin versus venlafaxine extended-release (XR) from a Spanish healthcare perspective. Microsimulation techniques, including Hamilton Anxiety Scale (HAM-A) score, number of weeks with minimal or no anxiety (HAM-A ≤ 9), and quality-adjusted life-years (QALYs), were used to predict treatment outcomes for patients with moderate-to-severe GAD who would be treated with pregabalin vs venlafaxine XR. Expected levels of healthcare utilization and unit cost of care are derived from Spanish published sources. We express cost-effectiveness alternatively in terms of incremental cost per additional week with minimal or no anxiety, and incremental cost per QALY gained [in 2007 Euros (€)]. Considering costs of drug treatment only, the incremental cost [mean (95% confidence interval)] of pregabalin (vs venlafaxine XR) would be €96 (€86, €107) per additional week with minimal or no anxiety, and €32,832 (€29,656, €36,308) per QALY gained. When other medical care costs are considered, cost-effectiveness ratios decline to €70 (€61, €80) per additional week with no or minimal anxiety, and €23,909 (€20,820, €27,006) per QALY gained. We conclude that, using a new microsimulation model of the treatment of GAD, pregabalin appears to be cost-effective vs venlafaxine XR in a Spanish healthcare setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anxiety disorders are considered the most prevalent type of psychiatric disorder, with generalized anxiety disorder (GAD) being the most common in the primary care setting [1]. Large epidemiologic studies have reported lifetime rates of GAD ranging from 2.8 to 5.1% [2–4]. The most common symptoms of GAD are both physical and psychological. Somatic complaints include chest pain, irritable bowel symptoms, headache, hyperventilation, fatigue, insomnia, joint pain, and palpitations [1]. Psychological complaints typically include pervasive and uncontrollable persistent worry and tension about daily life events lasting more than 6 months. Patients with GAD are more likely than other patients to present with medical comorbidities, seek medical care, and undergo extensive medical testing (to rule out other pathologies), making GAD a challenging medical condition to recognize [5, 6]. The burden of the disease is also notable in terms of restrictions on patients’ ability to carry out their daily activities. Reductions in patients’ health-related quality of life and well-being [1, 7] have been reported to be comparable in magnitude to those accompanying major depressive disorders.
Benzodiazepines have been shown to be useful for rapid, short-tem relief of somatic symptoms of GAD [8], and they are often used to help alleviate the restlessness associated with initiation of antidepressant therapy. Because of the potential for dependency, however, these agents are restricted to short-term use in many countries. Effective pharmacotherapies that may be used on a long-term basis in patients with GAD include antidepressants, such as paroxetine and escitalopram—both selective serotonin reuptake inhibitors (SSRIs)—and extended-release (XR) venlafaxine, a serotonin-norepineprhine reuptake inhibitor. If a patient’s initial response to treatment with these agents is positive, it is recommended that therapy be continued for 6 months to 1 year, and then tapered off [9]. As it is recognized that many patients with GAD are undertreated, and that the disease imposes a substantial economic burden on patients, for the healthcare system, and society at large, successful treatment of symptoms of GAD may confer substantial benefits. While the cost of chronic pharmacotherapy for GAD is not negligible, few formal economic evaluations of these agents have been reported in the published literature. The cost-effectiveness of venlafaxine XR (vs diazepam) was examined recently from the perspective of the United Kingdom’s National Health Service using a decision-analytic model [10]. The authors concluded that while first-line treatment with venlafaxine XR was more expensive, it was clinically more effective, reduced the overall cost of consultations to general practitioners and mental health-care providers, and was cost-effective in the management of non-depressed patients suffering from GAD. Another UK study suggested that first-line treatment with escitalopram may lead to higher treatment response rates and overall cost savings compared to first-line treatment with paroxetine [11].
In this study, we report on a new pharmacoeconomic model of GAD treatment that we developed to support medical decision-making in this patient population. To illustrate its use, we used the model to estimate the cost-effectiveness of pregabalin (an anticonvulsant agent indicated for the treatment of GAD in Europe) vs venlafaxine XR, using data from the Pregabalin Efficacy in Anxiety Clinical Evaluation (PEACE) trial [12] and resource utilization data from Spain.
Methods
Model overview
We developed a patient-level simulation model to estimate clinical and economic outcomes of pharmacotherapy in patients with GAD. A similar modeling approach has been employed in two prior published cost-effectiveness evaluations of pregabalin in the treatment of neuropathic pain and epilepsy, respectively [13, 14]. Patients in the model were assumed to have moderate-to-severe chronic anxiety at therapy initiation, consistent with the characteristics of study subjects in most clinical trials in this patient population. To characterize anxiety symptoms in patients with GAD, the model uses the Hamilton Anxiety Scale (HAM-A) [15]. The HAM-A is a clinician-rated symptom scale designed to quantify the severity of anxiety symptoms and to assess response to therapeutic interventions. The instrument consists of 14 items (anxious mood, tension, fears, insomnia, intellectual impairment, depressed mood, somatic muscular and sensory complaints, cardiovascular, respiratory, genitourinary and autonomic symptoms, and patient’s behavior at interview), each defined by a series of symptoms. Each item is scored on a 0–4 scale. The possible score range on the HAM-A is therefore 0–56.
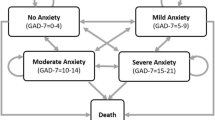
In the model, we allow HAM-A scores to vary with treatment from patient to patient. The model designates HAM-A scores ≤9 as “no or minimal anxiety”; 10–15, as “mild anxiety”; 16–24, as “moderate anxiety”; and ≥25, as “severe anxiety”. While (to the best of our knowledge) these categorizations have not been validated clinically, they have been employed by others [16, 17]. The value of using time without symptoms to compare treatments of chronic symptomatic diseases has long been recognized in a variety of medical conditions, including pain, depression, and epilepsy [18–22].
The model focuses attention on a hypothetical cohort of 1,000 patients with GAD, and simulates their symptoms of anxiety (as measured by their HAM-A scores) on a weekly basis up to 1 year (shorter time horizons also may be employed in the model). In the model, patients are first assigned an average pre-treatment HAM-A score, based on the distribution of HAM-A scores in the clinical trial (Fig. 1). The model then projects, for each patient in the cohort, the impact of therapy on weekly HAM-A scores, based on the expected change from baseline. Expected changes in HAM-A scores are permitted to vary from week to week (e.g., they may be lower during the first weeks following initiation of therapy); they also are permitted to vary from patient to patient, based on assumed variability of the weekly percentage change in HAM-A scores with treatment. For each treated patient, expected HAM-A scores are derived by multiplying the pretreatment HAM-A score by the expected percentage change in HAM-A score by week. After expected weekly HAM-A scores are assigned to each patient in the model, health-state utilities are then assigned for each week. The model considers the impact of therapy on GAD symptoms only; the potential effect of treatment on concomitant depressive symptoms, if present, is not considered. The impact of treatment on lost productivity due to GAD-related disability is also (conservatively) not considered.
The model allows for the possibility that patients may discontinue therapy if they experience lack of efficacy and/or side effects. If therapy discontinuation is permitted, patients may switch to another treatment or remain untreated. Patients also may be assumed to initiate care with their primary care provider or a specialist, and receive additional health-care services (e.g., specialists visits, laboratory tests, inpatient).
The model calculates a variety of summary measures of patient outcome, including the expected HAM-A score with treatment (mean over time), the expected number of weeks with no or minimal anxiety (HAM-A ≤ 9), mild anxiety (10–15), moderate anxiety (16–24), and severe anxiety (≥25), respectively, and quality-adjusted life-years (QALYs). These measures of clinical outcome are then combined with estimates of utilization and cost (of pharmacotherapy and, optionally, healthcare services) to calculate the cost-effectiveness of the treatments considered. The perspective of the analyses is that of a third-party payer. Utilization of healthcare services is assumed in the model to vary in relation to HAM-A scores. Cost-effectiveness is calculated alternatively in terms of the incremental cost per additional week with no or minimal anxiety, and the incremental cost per QALY gained.
To illustrate use of the model, we undertook an analysis comparing pregabalin and venlafaxine XR. Pregabalin is an anticonvulsant agent ([S]-3-[aminomethyl]-5-methylhexanoic acid) that is approved in Europe for the treatment of GAD; venlafaxine XR is a widely used SNRI that has shown substantial efficacy in patients with anxiety disorders. We based this comparison on data from the PEACE study [12], an 8-week, three-arm, multicenter, randomized, double-blind clinical trial of pregabalin (300–600 mg/day), venlafaxine XR (75–225 mg/day), and placebo in patients with GAD that was designed to address onset of efficacy, and which used flexible dosing schemes to reflect conditions of typical clinical practice. Approximately 130 patients were randomized in equal proportions to the three treatment groups. Mean (± SD) daily dose was 348 (± 85) mg for pregabalin and 102 (± 33) mg for venlafaxine. Versus placebo, pregabalin resulted in a statistically significant decrease from baseline to endpoint in HAM-A scores (P = 0.028); the difference between venlafaxine XR and placebo was not statistically significant (the trial was not powered to detect a difference between the two active comparators). Pregabalin and venlafaxine were both generally well tolerated.
Model estimation
At model entry, each patient in the hypothetical cohort was randomly assigned an initial HAM-A score by sampling (with replacement) from the actual distribution of HAM-A scores at study entry in the PEACE trial (data on file, Pfizer). Based on this assignment, 25% of patients were estimated to have moderate anxiety (HAM-A score 16–24) at study entry; the remainder were estimated to have severe anxiety (HAM-A score ≥25) (Table 1).
The expected weekly percentage changes in the HAM-A score over 8 weeks with pregabalin and venlafaxine XR were obtained by sampling (with replacement) from the distribution of HAM-A scores during follow-up among subjects in the PEACE study who were randomized to receive these agents (data on file, Pfizer); results are summarized in Table 2. As data were not available beyond 8 weeks, treatment benefit (i.e., mean percentage change in HAM-A) was assumed to be maintained from week 8 until 1 year. Alternative time horizons (8 weeks, 6 months) were examined in sensitivity analyses. The distribution of the change in HAM-A score with therapy was assumed to be left-truncated normal (i.e., percentage reduction in HAM-A with therapy could not be higher than 100%), based on empirical observation of patient-level data from the clinical trial.
In our basecase analyses, we assumed that patients would not discontinue therapy irrespective of its efficacy or the occurrence of side effects. In sensitivity analyses, we assumed that patients might discontinue therapy for these reasons, based on data from the PEACE study (Table 3). All patients who were assumed to discontinue therapy in sensitivity analyses were assumed to switch to treatment with paroxetine 20 mg/day, and to remain on such therapy for the remainder of time in the model. Alternative assumptions (0, 25, 50%) with respect to the rate of switching to paroxetine were examined in sensitivity analyses. All patients switching to paroxetine were assigned a mean weekly percentage change of 50%, based on published data [23–26].
Health-state utilities were assigned to patients on a weekly basis, based on their predicted severity of anxiety (i.e., no or minimal, mild, moderate, severe), using values from the EQ-5D Weighted Health Index (WHI) [27] obtained from a cross-sectional study of 456 patients with GAD from a representative randomly selected sample of 134 primary health centers in Spain [28]. Utility values were estimated in that study in relation to the severity of anxiety symptoms (as measured by HAM-A scores) (Table 3).
The costs of pregabalin (Lyrica®) and venlafaxine XR (Vandral Retard®) therapy were estimated using published price lists [29], assuming average daily dosages of 348 mg and 102 mg, respectively, based on data from the PEACE study; the reference price was employed for paroxetine (20 mg/day). The daily dosages of these medications are consistent with those recommended in product labeling information in Spain. Expected levels of healthcare utilization were derived from the above-described cross-sectional study, and included use of routine outpatient visits (primary care, specialists, other providers), emergency room visits, selected laboratory tests, and inpatient days (Table 4). Utilization of healthcare services in the study was estimated in relation to the severity of anxiety symptoms (as measured by HAM-A scores). Estimates of the costs of services were derived from a publicly available Spanish database [30] (Table 5). All costs are reported in 2007 Euros (€).
Analyses
The model was estimated using Monte Carlo simulation techniques [31, 32], assuming a cohort of 1,000 patients with moderate-to-severe GAD symptoms. During simulation, each patient was randomly stepped through the model, one at a time, yielding expected values for each patient in the cohort; patients were assumed to be treated alternatively with pregabalin and venlafaxine XR. Summarization of the outcomes of interest across all patients in the cohort yielded expected values for each treatment group. Measures of clinical outcome were then combined with estimates of the cost of pharmacotherapy and medical-care services to calculate the cost-effectiveness of pregabalin versus venlafaxine XR. Cost-effectiveness was examined alternatively in terms of the incremental cost per additional week with no or minimal anxiety, and the incremental cost per QALY gained. Incremental cost-effectiveness was examined under two alternative primary scenarios—one that considered only the cost of pharmacotherapy, and another that included costs of medical-care services along with the cost of pharmacotherapy.
Deterministic one-way sensitivity analyses were undertaken by varying selected assumptions and parameter estimates for which probability distributions were unknown or inapplicable, including: (1) modeling time horizon; (2) therapy discontinuation due to lack of efficacy and/or side effects; (3) rates of switching to paroxetine; (4) health-state utilities by HAM-A interval (alternatively, 25 and 75% quartile values from the Spanish cross-sectional study, and EQ-5D values from the PEACE clinical trial); (5) utilization of medical-care services; and (6) the costs of medical-care services. One-way sensitivity analyses were conducted for the incremental cost per QALY gained only.
Probabilistic sensitivity analyses (i.e., second-order Monte Carlo simulations) were undertaken to address uncertainty with respect to the weekly mean percentage changes in HAM-A scores with pregabalin and venlafaxine XR respectively. We ran the model for 100 samples of 1,000 patients each in these analyses. A left-truncated normal distribution was assumed for the weekly expected percentage changes in the HAM-A during simulation (i.e., percentage mean reduction in HAM-A with therapy could not be higher than 100%, based on empirical observation of patient-level data from the clinical trial). Cost-effectiveness acceptability curves reflecting the probability that pregabalin would be cost-effective (vs venlafaxine XR) at various thresholds of willingness to pay also were generated under alternative analytical scenarios.
Results
Estimates of expected clinical outcomes and costs in patients with GAD treated with pregabalin vs venlafaxine XR, respectively, are reported in Table 6. The average HAM-A score at therapy initiation was estimated to be 27.1; the estimated mean HAM-A score at the end of 1 year was 10.6 for patients treated with pregabalin and 12.8 for those treated with venlafaxine XR. The expected total number of weeks (over 1 year) with no or minimal anxiety (HAM-A ≤9) was 13.5 for pregabalin and 4.3 for venlafaxine XR. Pregabalin was also estimated to yield reductions in the number of weeks with mild anxiety (HAM-A 10–15), moderate anxiety (HAM-A 16–24), and severe anxiety (HAM-A ≥25). The estimated mean number of QALYs gained with pregabalin therapy (over 1 year) was 0.027 (0.740 vs 0.713 for venlafaxine XR).
The mean cost of pharmacotherapy over 1 year was estimated to be €1,664 for pregabalin, and €780 for venlafaxine XR. Mean costs of medical-care services were estimated to be €2,207 for patients receiving pregabalin and €2,454 for those receiving venlafaxine XR; the difference was attributable to expected differences in visits to primary care physicians, mental health-care providers, laboratory tests, and inpatient days. Total estimated costs over 1 year were €3,871 for pregabalin and €3,234 for venlafaxine XR.
The incremental cost of pregabalin therapy (vs venlafaxine XR) per additional week with no or minimal anxiety was [mean (95% CI)] €96 (€86, €107) (Table 7) when pharmacotherapy costs only were considered; the corresponding incremental cost per QALY gained was €32,832 (€29,656, €36,308). When costs of all medical-care services were included, corresponding ratios were €70 (€61, €80) per additional week with no or minimal anxiety, and €23,909 (€20,820, €27,006) per QALY gained.
Findings from one-way deterministic sensitivity analyses on key model assumptions and parameter estimates are reported in Table 8. In these analyses, the incremental cost per QALY gained for pregabalin therapy ranged from €21,836 to €67,928 under varying assumptions. The ratio was low when other cost offsets were included, when therapy discontinuation was allowed, and when patients were assumed to return to an untreated health state, and when 75% quartile values were employed for health-state utilities for each of the model defined HAM-A score intervals (cost of pharmacotherapy only); it was highest when the modeling time horizon was limited to 8 weeks (the duration of follow-up in the PEACE trial). Ratios also increased when 25% quartile values were employed as estimates of health-state utilities. Estimates were also sensitive to changes in the assumed rate of switching to paroxetine when assumptions of therapy discontinuation were employed. Findings were not particularly sensitive to increases in the assumed risk of therapy discontinuation due to side effects or lack of efficacy, the use of a 6-month modeling time horizon, and changes in estimates of resource utilization and the unit costs of medical-care services (cost offsets scenario). In probabilistic sensitivity analyses, estimates of the cost-effectiveness of pregabalin (vs venlafaxine XR) were in the vicinity of €30,000 per QALY gained, an arbitrarily accepted threshold value for willingness to pay [33] (Fig. 2).
Discussion
In this paper, we report on a simulation model that we developed to estimate expected clinical and economic outcomes associated with the treatment of GAD, which may be used to support therapeutic decision making in this patient population. When we used our model to examine the cost-effectiveness of pregabalin vs venlafaxine XR in the treatment of GAD in a Spanish setting, we found that pregabalin would cost an estimated €96 (€86, €107) per additional week with no or minimal anxiety, and €32,832 (€29,656, €36,308) per QALY gained (both over 1 year). When estimates of potential offsets in the utilization of medical-care services were included, corresponding estimates were €70 (€61, €80) per additional week with no or minimal anxiety, and €23,909 (€20,820, €27,006) per QALY gained. The incremental cost per QALY gained was €30,000 or less across most scenarios in sensitivity analyses.
To the best of our knowledge, only two prior studies have used models to examine the cost-effectiveness of pharmacotherapy in patients with GAD [10, 11]; both were conducted in the United Kingdom (UK). Differences in analytical approach, outcome measures, time horizons, and assumptions employed in these two studies preclude comparisons with our findings. Guest and colleagues [10] examined the cost-effectiveness of venlafaxine XR vs diazepam over 6 months from the perspective of the UK’s National Health Service. They used a deterministic decision-analytic model and reported an estimate of the incremental cost per additional patient successfully treated with venlafaxine XL (vs diazepam) (GPB £380), and the incremental cost for each additional patient in whom a relapse would be avoided (£295) (both in 2000/2001 prices); they concluded that starting treatment with venlafaxine rather than diazepam was more effective clinically and more cost-effective for managing non-depressed patients with GAD in the UK. The Investigator’s Clinical Global Impression (CGI) improvement score was used as the key clinical measure in the model, but the authors recognized that the CGI might be less robust than HAM-A scores. Jorgenssen and colleagues [11] used a similar model and reported higher rates of first-line treatment success and lower rates of discontinuation due to adverse events over 9 months for patients treated with escitalopram (vs paroxetine), as well as cost savings of £1,408 (at 2004 price levels) from a societal perspective. Treatment success and relapse were defined in the model using the CGI alone or in combination with HAM-A threshold values and therapy discontinuation due to lack of efficacy. The authors did not report estimates of the cost-effectiveness of escitalopram vs paroxetine. In both models, assumptions of therapy discontinuation and switching to another pharmacotherapy for GAD (i.e., paroxetine, diazepam) were employed.
A few key aspects of our model should be noted. One particular strength is its stochastic (as opposed to deterministic) nature, which takes into consideration the uncertainty inherent in estimates of the average change in HAM-A scores with treatment. Second, measures of clinical effectiveness employed in our model include time (i.e., weeks) with no or minimal anxiety in addition to QALYs. While use of QALYs allows comparisons with findings from pharmacoeconomic evaluations of interventions for other medical conditions, use of “time without symptoms” may best reflect favorable clinical outcomes from the patient’s perspective [18–22]. We believe that our study is the first to report estimates of the cost-effectiveness of pharmacologic treatment of GAD using such a measure. Third, while including all relevant costs and consequences of therapy in health-economic evaluations is often recommended, successful treatment is often assumed to result in reduced utilization of medical-care services (and sometimes indirect cost savings), which typically offset the cost of the intervention. Because we believe there is significant uncertainty with respect to the expected impact of effective pharmacotherapy on GAD-related medical-care services, our model permits examination of economic benefits alternatively considering the cost of pharmacotherapy only and including potential offsets in the utilization and cost of other medical-care services. Taken together, these estimates probably represent reasonable “conservative” and “liberal” boundaries on the economic impact of pharmacotherapy for GAD.
Important limitations of our approach also should also be noted. First, the maximum time horizon in our model is 1 year. During model development, we thought that we could neither establish a reasonable treatment algorithm for patients over a longer time horizon (e.g., 5 years, lifetime), nor accurately estimate the expected change in HAM-A scores with follow-on therapy conditional upon success or failure of prior therapies over time. Use of a 1-year time horizon is also consistent with current recommendations regarding the treatment of GAD (i.e., 6 months–1 year, and then taper) [9]. Our model, however, permits examination of cost-effectiveness for shorter time horizons, including the typical duration of clinical trials (e.g., 8–12 weeks). Second, because the duration of follow-up in the PEACE study was 8 weeks, and in the absence of long-term data, we assumed that the efficacy of pregabalin and venlafaxine XR observed in the trial would be maintained over 1 year; this may or may not be an accurate depiction of reality. Third, in our primary analyses, patients were assumed to be treated over 1 year assuming without treatment discontinuation due to lack of efficacy or side effects. This approach obviated the need to specify a follow-on treatment strategy. While this may be unrealistic, we believe it provides a framework for interpretation of the benefits of the therapies of interest that is not confounded by assumptions regarding the sequence of subsequent therapies that patients might receive and their efficacy in this context. However, we did examine the impact of therapy discontinuation and alternative switching rates in sensitivity analyses. The cost-effectiveness of pregabalin therapy improved somewhat when we assumed that therapy discontinuation due to lack of efficacy and/or side effects would occur, suggesting that estimates from our primary analyses were conservative.
In summary, we believe the analytical model developed here can be of value in considering the cost-effectiveness of alternative treatments for patients with moderate-to-severe GAD. Our findings also suggest that, from a Spanish perspective, pregabalin may be cost-effective in comparison with venlafaxine XR in the treatment of such patients.
References
Wittchen, H.U., Kessler, R.C., Beesdo, K., et al.: Generalized anxiety and depression in primary care: prevalence, recognition, and management. J. Clin. Psychiatr. 63(Suppl. 8), 24–34 (2002)
Jenkins, R., Lewis, G., Bebbington, P., et al.: The National Psychiatric Morbidity surveys of Great Britain—initial findings from the household survey. Psychol. Med. 27(4), 775–789 (1997). doi:10.1017/S0033291797005308
Lieb, R., Becker, E., Altamura, C.: The epidemiology of generalized anxiety disorder in Europe. Eur. Neuropsychopharmacol. 15(4), 445–452 (2005). doi:10.1016/j.euroneuro.2005.04.010
Kessler, R.C., Wang, P.S.: The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu. Rev. Public Health 29, 115–129 (2008). doi:10.1146/annurev.publhealth.29.020907.090847
Katon, W., von Korff, M., Lin, E., et al.: Distressed high utilizers of medical care: DMS-III-R diagnoses and treatment needs. Gen. Hosp. Psychiatr. 12(6), 35–62 (1990). doi:10.1016/0163-8343(90)90002-T
Kennedy, B.L., Schwab, J.J.: Utilization of medical specialists by anxiety disorder patients. Psychosomatics 38(2), 109–112 (1997)
Hoffman, D.L., Dukes, E., Wittchen, H.U.: Human and economic burden of generalized anxiety disorder. Depress. Anxiety 25(1), 72–90 (2008). doi:10.1002/da.20257
Rickels, K., Downing, R., Schweizer, E., Hassman, H.: Antidepressants for the treatment of generalized anxiety disorder: a placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch. Gen. Psychiatr. 50(11), 884–895 (1993)
Fricchione, G.: Generalized anxiety disorder. N. Engl. J. Med. 351(7), 675–682 (2004). doi:10.1056/NEJMcp022342
Guest, J.F., Russ, J., Lenox-Smith, A.: Cost-effectiveness of venlafaxine XL compared with diazepam in the treatment of generalized anxiety disorder in the United Kingdom. Eur. J. Health Econ. 6, 136–145 (2005). doi:10.1007/s10198-004-0272-z
Jorgenssen, T.R., Stein, D.J., Despiegel, N., et al.: Cost-effectiveness of escitalopram compared with paroxetine in treatment of generalized anxiety disorder in the United Kingdom. Ann. Pharmacother. 40, 1752–1758 (2006). doi:10.1345/aph.1H156
Baldinetti, F., Bandelow, B., Kasper, S., Herman, B., Nivoli, G., Van Ameringen, M., Petralia, A., Mandel, F.S.: Efficacy of pregabalin and venlafaxine-XR in generalized anxiety disorder: results of a double-blind, placebo-controlled 8-week trial. Int. Clin. Psychopharmacol. 24, 87–96 (2009)
Vera-Llonch, M., Brandenburg, N, Oster, G: Cost-effectiveness of pregabalin in patients with partial refractory epilepsy. Epilepsia 49(3), 431–437 (2008). doi:10.1111/j.1528-1167.2007.01279.x
Vera-Llonch, M., Dukes, E., Delea, T., Wang, S.T., Oster, G.: Treatment of peripheral neuropathic pain: a dynamic simulation model. Eur. J. Pain 10(3), 279–285 (2006). doi:10.1016/j.ejpain.2005.05.005
Hamilton, M.: The assessment of anxiety states by rating. Br. J. Med. Psychol. 32(1), 50–55 (1959)
Revicki, D., Brandenburg, M., Matza, L.S., et al.: Relationship between anxiety severity and health utility index scores in generalized anxiety disorder. Collegium of Internationale Neuro-Psycharmacologicum (CINP), Chicago, IL, USA, 9–13 July 2006
Morlock, R., Shah, H., Feltner, D., Brandenburg, N., Matza, L.S., Revicki, D.: Estimation of health-state utilities for patients with generalized anxiety disorder. Collegium of Internationale Neuro-Psycharmacologicum (CINP), Chicago, IL, USA, 9–13 July 2006
Feldstein, M.L.: Quality-of-life-adjusted survival for comparing cancer treatments. A commentary on TWiST and Q-TWiST. Cancer 67(Suppl 3), 851–854 (1991). doi:10.1002/1097-0142(19910201)67:3+<851::AID-CNCR2820671417>3.0.CO;2-G
Einarson, T.R., Arikian, A., Iskedjian, M.: Pharmacoeconomic analyses of venlafaxine in the treatment of major depressive disorder. Pharmacoeconomics 12(2 Pt 2), 286–296 (1997). doi:10.2165/00019053-199712020-00019
Markowitz, M.A., Mauskopf, J.A., Halpern, M.T.: Cost-effectiveness model of adjunctive lamotrigine for the treatment of epilepsy. Neurology 51(4), 1026–1033 (1998)
Blais, L., Sheehy, O., Saint-Hilaire, J.M., Bernier, G.P., Godfroid, P., LeLorier, J.: Economic evaluation of levetiracetam as an add-on therapy in patients with refractory epilepsy. Pharmacoeconomics 23(5), 493–503 (2005). doi:10.2165/00019053-200523050-00008
Wan, G.W., Zhang, H.F., Tedeschi, M.A., Hackett, D.: Estimation of symptom-free days in generalized anxiety disorders. Curr. Med. Res. Opin. 22(3), 587–591 (2006). doi:10.1185/030079906X96245
Ball, S.G., Kuhn, A., Wall, D., et al.: Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J. Clin. Psychiatr. 66, 94–99 (2005)
Rickels, K., Zaninelli, R., McCafferty, J., Bellew, K., Iyengar, M., Sheehan, D.: Paroxetine treatment of generalized anxiety disorder: a double-blind, placebo-controlled study. Am. J. Psychiatr. 160, 749–756 (2003). doi:10.1176/appi.ajp.160.4.749
Pollack, M.H., Zaninelli, R., Goddard, A., et al.: Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatr. 62, 350–357 (2001)
Rocca, P., Fonzo, V., Scotta, M., Zanalda, E., Ravizza, L.: Paroxetine efficacy in the treatment of generalized anxiety disorder. Acta Psychiatr. Scand. 95, 444–450 (1997). doi:10.1111/j.1600-0447.1997.tb09660.x
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35(11), 1095–1108 (1997). doi:10.1097/00005650-199711000-00002
Rovira, J., Albarracin, G., Salvador-Carulla, L., Rejas, J.: Cost and burden of illness of generalized anxiety disorder (GAD): a Spanish perspective from the primary health-care setting (The ANCORA Study). Eur. J. Neuropsychopharmacol. 17(Suppl 4), S507–S508 (2007). doi:10.1016/S0924-977X(07)70781-2
Catalogo del Consejo General de Colegios Farmaceuticos: Base de datos de medicamentos. Consejo General de Colegios Oficiales de Farmaceuticos. http://www.portalfarma.com/home.nsf (accessed March 2008)
Oblikue Consulting 2007. http://www.oblikue.com/bddcostes (accessed March 2008)
Craig, B., Black, M., Sendi, P.: Uncertainty in decision models analyzing cost-effectiveness. Med. Decis. Making 20(1), 135–137 (2000). doi:10.1177/0272989X0002000118
Halpern, E., Weinstein, M., Hunink, M., Gazelle, G.S.: Representing first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med. Decis. Making 20(3), 314–322 (2000). doi:10.1177/0272989X0002000308
Sacristan, J.A., Oliva, J., Del Llano, J., Prieto, L., Pinto, J.L.: Que es una tecnologia sanitaria eficiente en Espana? Gac. Sanit. 16(4), 334–343 (2002)
Acknowledgments
Financial support for this study was provided by Pfizer, Inc. M. V.-L., O. S., and G. O. are employees of Policy Analysis Inc., who were paid consultants to Pfizer in connection with the development of this manuscript. E. D., M. M., and J. R. are employees of Pfizer, Inc.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Vera-Llonch, M., Dukes, E., Rejas, J. et al. Cost-effectiveness of pregabalin versus venlafaxine in the treatment of generalized anxiety disorder: findings from a Spanish perspective. Eur J Health Econ 11, 35–44 (2010). https://doi.org/10.1007/s10198-009-0160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-009-0160-7





