Abstract
Introduction
Patients with moderate to severe allergic asthma have persistent poorly controlled asthma despite inhaled or systemic corticosteroid therapy. New therapies are becoming more widely available to treat such patients, but their value needs to be formally assessed in an economic evaluation. Within a publicly funded health care system such an analysis should reflect societal preferences when measuring treatment benefits. The aim of this study was to elicit societal preferences for the symptom burden associated with moderate to severe allergic asthma.
Method
Existing daily symptom diary data from a clinical trial were used to develop health state descriptions for evaluation in a standard gamble interview. Five health states were produced that reflected five distinct levels of control ranging from ‘complete control of asthma’ to ‘worsening of asthma’, as defined by another outcome measure. The symptom diary data were also used as attributes in a discrete choice experiment (DCE) to estimate willingness to pay for improvements in symptoms. Members of the general public (n = 101) completed the interview.
Results
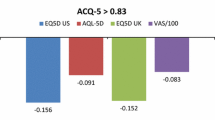
Thirteen participants failed the consistency checks and were excluded from the analysis. Societal utility ratings for the health states ranged from 0.71 (worsening of asthma) to 0.78 (complete control of asthma). The participants were also willing to pay £160 a month for the avoidance of all symptoms.
Conclusions
The range of utility values (0.71–0.78) demonstrates the severity of moderate to severe allergic asthma. However the spread of scores between complete control of asthma and worsening of asthma was lower than was expected. The community sample placed only a moderate value on the avoidance of all asthma symptoms in the DCE survey. The results suggest that the community sample may not have fully understood the benefits of control over asthma symptoms and the limitations such symptoms can impose on everyday life.

Similar content being viewed by others
References
Bennett, K., Torrance, G.: Measuring health state preferences and utilities: rating scale, time trade-off and standard gamble techniques. In: Spilker, B. (eds.) HRQL and Pharmacoeconomics in Clinical Trials, 2nd edn. Lippincott-Raven, Philadelphia, pp. 253–266 (1996)
Busse, W., Corren, J., Quentin Lanier, B., McAlary, M., Fowler-Taylor, A., Della Cioppa, van As A., Gupta, N.: Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 108, 184–190 (2001)
Conner-Spady, B.L., et al.: A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 36(3), 251–259 (2005)
Finn, A., Gross, G., van Bavel, J., Lee, T., Windom, H., Everhard, F., Fowler-Taylor, A., Liu, J., Gupta, N.: Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J. Allergy Clin. Immunol. 111, 278–284 (2003)
Furlong, W., Feeny, D., Torrance, G.W., Barr, R.D., Horsman, J.: Guide to design and development of health-state utility instrumentation. McMaster University CHEPA working paper series (1990)
Global Initiative for Asthma (GINA): Global strategy for asthma management and prevention. NIH Publication 02-3659 issued January 1995 (updated 2002, 2003; accessed 26 Oct 2004). Available at: http://www.ginasthma.com
Gold, M.R., Siegel, J.E., Russell, L.B., Weinstein, MC.: Cost-Effectiveness in Health and Medicine. Oxford University Press (1996)
Greene, W.H., Greene, L.K., Seaks, T.G.: Estimating the functional form of the independent variables in probit models. Appl. Econ. 27, 193–196 (1995)
Humbert, M., Beasley, R., Ayres, J., Slavin, R., Hebert, J., Bousquet, J., Beech, K-M., Ramos, S., Canonica, G.W., Hedgecock, S., Fox, H., Blogg, M., Surrey, K.: Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Asthma 10, 1398 (2004)
Kind, P., Dolan, P., Gudex, C., Williams, A.: Variations in population health status: results from a United Kingdom national questionnaire survey. Br. Med. J. 316, 736–741 (1998)
Lloyd, A.J., McIntosh, E., Rabe, K., Williams, A.E.: Patient preferences for asthma therapy: a discrete choice experiment. Prim. Care Respir. J. (in press)
Lloyd, A.J., Price, D., Brown, R.: The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim. Care Respir. J. 16, 22–27 (2007)
Lloyd, A., Turk, F., Leighton, T., Canonica, G.W.: Psychometric evaluation of global evaluation of treatment effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J. Med. Econ. 10(3), 285–296 (2007)
Louviere, J., Hensher, D., Swait, J.: Conjoint preference elicitation methods in the broader context of Random Utility Theory preference eliciation methods. In: Gustafson, A., Hermann, A., Huber, F. (eds.) Conjoint Measurement: Methods and Applications. Springer, Berlin, pp. 305–344 (2001)
Manski, C.F.: The structure of random utility models. Theory Decis. 8(229), 254 (1977)
Marshall. G.D. Jr, Sorkness, C.A.: IgE-blocking therapy for difficult-to-treat asthma: a brief review. Manag. Care 13(3), 45–50 (2004)
McFadden, E.R. Jr: Acute severe asthma. Am. J. Respir. Crit. Care Med. 168(7), 740–759 (2003)
Norum, J., et al.: Treatment costs in Hodgkin’s disease: a cost-utility analysis. Eur. J. Cancer 32A(9), 1510–1517 (1996)
Office of National Statistics, National Statistics website “Population of the United Kingdom: by ethnic group, April 2001”. http://www.statistics.gov.uk/cci/nugget_print.asp?ID=764
Osman, L.M., McKenzie, L., Cairns, J., Friend, J.A.R., Godden, D.J., Legge, J.S., Douglas, J.G.: Patient weighting of importance of asthma symptoms. Thorax 56, 138–142 (2001)
Roe, B., Boyle, K.J., Teisl, M.F.: Using conjoint analysis to derive estimates of compensating variation. J. Environ. Econ. Manage. 31, 145–159 (1996)
Solèr, M., Matz, J., Townley, R., Buhl, R., O’Brien J., Fox, H., Thirlwell, J., Gupta, N.,Della Cioppa, G.: The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur. Respir. J. 18, 254–261 (2001)
Torrance, G.W.: Measurement of health state utilities for economic appraisal. J. Health Econ. 5(1), 1–30 (1986)
Torrance, G.W.: Preferences for health states: a review of measurement methods. Clin. Econ. Eval. Perinat. Dev. Med. 20, 37–45 (1982)
Torrance, G.W.: Utility approach to measuring health-related quality of life. J. Chronic Dis. 40(6), 593–600 (1987)
Acknowledgments
The authors would like to acknowledge the assistance of Bernadette Khoshaba who helped to develop the health states and interviewed some study participants. The study was funded by Novartis AG.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Complete control of asthma
Marked improvement of asthma
Discernible, but limited improvement in asthma
No appreciable change in asthma
Worsening of asthma
Appendix 2: attributes and levels from DCE
Morning asthma symptoms
-
Each morning when you wake up you did not have any asthma symptoms (such as chest tightness, wheezing or cough).
-
Each morning when you wake up you have asthma symptoms (such as chest tightness, wheezing or cough).
Nighttime breathing problems
You may experience breathing problems at night. These normally include chest discomfort (tightness), wheezing, and cough, and possibly shortness of breath.
-
You did not wake up because of any breathing problems.
-
You wake up once a night because of breathing problems but did not need to use your inhaler.
-
You wake up once because of breathing problems, but you used your inhaler to control the symptoms.
-
You awoke more than once because of breathing problems, but you used your inhaler to control the symptoms.
Daytime asthma symptoms
You may experience asthma symptoms during the day. These include—shortness of breath (breathlessness), chest discomfort (tightness), wheezing, and cough.
-
You have no symptoms at all; unrestricted activity.
-
You have some symptoms which caused little or no discomfort; unrestricted activity.
-
You have symptoms which cause some discomfort, at times limiting strenuous activity.
-
You have symptoms which cause moderate discomfort and sometimes limited routine activity.
Cost
You would have to pay for your new asthma medication out of you pocket. Think about whether you could afford the price and whether you would be willing to pay.
-
Cost is £20 per month
-
Cost is £40 per month
-
Cost is £60 per month
-
Cost is £80 per month
Rights and permissions
About this article
Cite this article
Lloyd, A., Doyle, S., Dewilde, S. et al. Preferences and utilities for the symptoms of moderate to severe allergic asthma. Eur J Health Econ 9, 275–284 (2008). https://doi.org/10.1007/s10198-007-0075-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-007-0075-0