Abstract
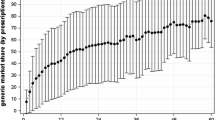
This study examined the relationship between generic drug market shares and the number of reported side effects. Yearly time-series data for the number of reported side effects and information on market shares, prices, and quantities from 1972 to 1996 were used in this study. Poisson and negative binomial regression models were used in the statistical analysis. The results show that increased generic market share increases the number of reported side effects for all estimated models. When studying the relationship at the substance level, increasing generic market shares increases the number of side effects for 7 of the 15 substances. Generic substitution laws and measures to increase generic competition may thus have unintended consequences since these results show a positive relationship between generic market shares and reported side effects.
Similar content being viewed by others
References
Ganguli R (2002) Rationale and strategies for switching antipsychotics. Am J Health Syst Pharm 59:522–526
Goldstein JM, Cantillion M (1998) Safety of switching to quetiapine furmarate. Presented at the 151th Annual Meeting of the American Psychiatric Association. Toronto, Canada, 30 May 30–4 June
Kinon BJ, Basson BR, Gilmore JA, Malcolm S, Stauffer VL (2000) Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. J Clin Psychiatry 61:833–840
Hartley K, Lavers RJ, Maynard AK (1986) Regulation and development times in the UK pharmaceutical industry. Scott J Polit Econ 33:355–369
Andersson F, Hertzman P (1993) Effective patent life of drugs in Sweden—a comparison with international studies. Manag Dec Econ 14:53–63
Rudholm N (2001) Entry and the number of firms in the Swedish pharmaceuticals market. Rev Ind Organ 19:351–364
Kendall KW, Ng S, Schoner B (1991) Consumer responses to generic/chemically equivalent drugs. J Public Policy Marketing, 10:182–201
Cameron AC, Trivedi P (1998) Regression analysis of count data. Cambridge University Press: Cambridge
Hausman JA, Hall BH, Griliches Z (1984) Econometric models for count data with an application to the patents—R and D relationship. Econometrica 52:909–938
LVFS (1996) 4 Läkemedelsverkets föreskrifter och allmäna råd om säkerhets-övervakning av läkemedel
SFS (1993) 1057 Förordning om viss uppgiftsskyldighet inom hälso-och sjuk-vården
SFS (1994) 1521 Förordning om biverkningsregister angående läkemedel
Acknowledgements
Financial support from the Jan Wallander and Tom Hedelius Foundation is gratefully acknowledged. The authors thank Jonas Bergwall, two anonymous referees, and the participants at a seminar at Umeå University for valuable comments and suggestions.
Conflict of interest:
No information supplied
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellström, J., Rudholm, N. Side effects of generic competition?. Eur J Health Econom 5, 203–208 (2004). https://doi.org/10.1007/s10198-004-0234-5
Issue Date:
DOI: https://doi.org/10.1007/s10198-004-0234-5