Abstract
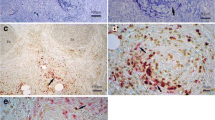
The aim of this study was to clarify the differences in the pathogenesis of neuropathy between myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA)-positive and -negative patients with Churg–Strauss syndrome (CSS). Eight MPO-ANCA-positive and 14 MPO-ANCA-negative patients were included. In addition to the standard histology, nerve biopsies were examined, employing immunohistochemistry for eosinophil major basic protein and electron microscopy. The groups did not differ significantly in clinical profiles, including the peak disability score and number of blood eosinophils. In nerve biopsies, necrotizing vasculitis was found in 63% (5/8) of the ANCA-positive and 21% (3/14) of the ANCA-negative patients. Fibrinoid necrosis of vessel walls was noted in 4 ANCA-positive patients (50%), and in one ANCA-negative patient (p = 0039). In contrast, a large number of eosinophilic infiltrations in the epineurium was shown in 36% (5/14) of the ANCA-negative patients, with no eosinophilic infiltrations shown in ANCA-positive patients. In 3 ANCA-negative patients, endoneurial eosinophils were seen where focal axonal loss and capillary dilatation were occasionally noted. There may be 2 pathogenetic mechanisms of neuropathy with CSS: ANCA-related vascular fibrinoid necrosis, and a toxic eosinophilic effect on nerve fibers which is independent of ANCA. Therapy targeting activated eosinophils may be a possible treatment for intractable neuropathy of CSS.

Similar content being viewed by others
References
Collins MP, Kissel JT. Neuropathies with systemic vasculitis. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. 4th ed. Philadelphia: Elsevier; 2005. p. 2335–404.
Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421–30.
Hattori N, Mori K, Misu K, Koike H, Ichimura M, Sobue G. Mortality and morbidity in peripheral neuropathy associated Churg–Strauss syndrome and microscopic polyangiitis. J Rheumatol. 2002;29:1408–14.
Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92.
Said G, Lacroix C. Primary and secondary vasculitic neuropathy. J Neurol. 2005;252:633–41.
Dyck PJ, Conn DL, Okazaki H. Necrotizing angiopathic neuropathy. Three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc. 1972;47:461–75.
Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094–100.
Vital A, Vital C, Viallard JF, Ragnaud JM, Canron MH, Lagueny A. Neuro-muscular biopsy in Churg–Strauss syndrome: 24 cases. J Neuropathol Exp Neurol. 2006;65:187–92.
Rothenberg ME. Mechanisms of disease: Eosinophilia. N Engl J Med. 1998;1338:1592–600.
Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137:2913–7.
Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–6.
Schmitt WH, Csernok E, Kobayashi S, Klinkenborg A, Reinhold-Keller E, Gross WL. Churg–Strauss syndrome: serum markers of lymphocyte activation and endothelial damage. Arthritis Rheum. 1986;41:445–52.
Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg–Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. 1999;78:26–37.
Sinico RA, Di Toma L, Maggiore U, Bottero P, Radice A, Tosoni C, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg–Strauss syndrome. Arthritis Rheum. 2005;52:2926–35.
Kallenberg CG. Churg–Strauss syndrome: just one disease entity? Arthritis Rheum. 2005;52:2589–93.
Sablé-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, et al. Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann Intern Med. 2005;143:632–8.
Guillain–Barré Syndrome Steroid Trial Group. Double-blind trial of intravenous methylprednisolone in Guillain–Barré syndrome. Lancet. 1993;341:586–90.
Kaji R, Oka N, Tsuji T, Mezaki T, Nishio T, Akiguchi I, et al. Pathological findings at the site of conduction block in multifocal motor neuropathy. Ann Neurol. 1993;33:152–8.
Oka N, Kawasaki T, Mizutani K, Sugiyama H, Akiguchi I. Hypoxia-inducible factor 1-alpha may be a marker for vasculitic neuropathy. Neuropathology. 2007;27:509–15.
Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Nephrol Dial Transplant. 1998;13(Suppl 1):16–20.
Wiik A. Clinical and pathophysiological significance of anti-neutrophil cytoplasmic autoantibodies in vasculitis syndromes. Mod Rheumatol. 2009;19:590–9.
Bajema IM, Hagen EC, Heer E, VanDerWoude FJ, Bruijn JA. Colocalization of ANCA-antigens and fibrinoid necrosis in ANCA-associated vasculitis. Kidney Int. 2001;60:2025–30.
Vital C, Vital A, Canron MH, Jaffré A, Viallard JF, Ragnaud JM, et al. Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst. 2006;11:20–9.
Nagashima T, Cao B, Takeuchi N, Chuma T, Mano Y, Fujimoto M, et al. Clinicopathological studies of peripheral neuropathy in Churg–Strauss syndrome. Neuropathology. 2002;22:299–307.
Tsurikisawa N, Taniguchi M, Saito H, Himeno H, Ishibashi A, Suzuki S, et al. Treatment of Churg–Strauss syndrome with high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2004;92:80–7.
Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20.
von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–11.
Acknowledgments
This work was supported by grants from the Japanese Ministry of Health, Labour and Welfare.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oka, N., Kawasaki, T., Matsui, M. et al. Two subtypes of Churg–Strauss syndrome with neuropathy: the roles of eosinophils and ANCA. Mod Rheumatol 21, 290–295 (2011). https://doi.org/10.1007/s10165-010-0400-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-010-0400-9