Abstract
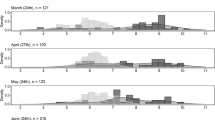
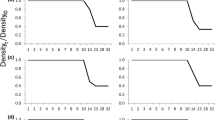
Larvae of the salamander, Hynobius retardatus, are carnivorous, and even though there are two morphs, a typical morph and a broad-headed or “cannibal” morph, both are cannibalistic. They also sometimes eat other large prey, for example larvae of the frog, Rana pirica. In natural habitats, use of both conspecific and R. pirica larvae as food may contribute more strongly to high survival and substantially to fitness when larval densities are higher, because early-stage H. retardatus larvae sometimes experience scarcity of their typical prey. In cannibalistic oviparous amphibians, larger individuals that developed from larger eggs can more efficiently catch and consume larger prey and thus their survival may be better than that of smaller individuals developed from smaller eggs. Populations might therefore diverge in respect of egg size in response to variation in the density of conspecific and R. pirica larvae in natural ponds, with eggs being larger when larval density is higher. I examined how variance in hatchling size correlated with the incidence of cannibalism, and whether increasing larval density in natural ponds correlated with increasing egg size. Variance in initial larval body size facilitated cannibalism, and egg size increased as larval density in the ponds increased. In ponds with high larval density, where cannibalism and large prey consumption is a critical factor in offspring fitness, the production of fewer clutches with larger eggs, and thus of fewer and larger offspring, results in greater maternal fitness. Variation among the mean egg size in populations is likely to represent a shift in optimum egg size across larval density gradients.



Similar content being viewed by others
References
Altwegg R, Reyer HU (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Azevedo RBR, French V, Partridge L (1997) Life-history consequences of egg size in Drosophila melanogaster. Am Nat 150:250–282
Bernardo J (1996) The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am Zool 36:216–236
Berven KA (1982) The genetic basis of altitudinal variation in the wood frog Rana sylvatica. An experimental analysis of larval development. Oecologia 52:360–369
Crump ML (1981) Variation in propagule size as a function of environmental uncertainty for tree frogs. Am Nat 117:724–737
Crump ML (1992) Cannibalism in amphibians. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford, New York, pp. 256–276
Einum S, Fleming JA (1999) Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc R Soc Lond B 266:2095–2100
Fox CW, Thakar MS, Mousseau TA (1997) Egg size plasticity in a seed beetle: an adaptive maternal effect. Am Nat 149:149–163
Goater CP (1994) Growth and survival of postmetamorphic toads: interactions among larval history, density, and parasitism. Ecology 75:2264–2274
Kaplan RH (1980) The implications of ovum size variability for offspring fitness and clutch size within several populations of salamanders (Ambystoma). Evolution 34:51–64
Kaplan RH (1998) Maternal effects, developmental plasticity, and life history evolution. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford, New York, pp. 244–260
Kaplan RH, Cooper WS (1984) The evolution of developmental plasticity in reproductive characteristics: an application of the “adaptive coin-flipping” principle. Am Nat 123:393–410
Laugen A, Laurila A, Merila J (2002) Maternal and genetic contributions to geographical variation in Rana temporaria larval life-history traits. Biol J Linn Soc 76:61–70
Maret TJ, Collins JP (1994) Individual responses to population size structure: the role of size variation in controlling expression of a trophic polyphenism. Oecologia 100:279–285
McGinley MA, Temme DH, Geber MA (1987) Parental investment in offspring in variable environments: theoretical and empirical considerations. Am Nat 130:370–398
Michimae H (2006) Differentiated phenotypic plasticity in larvae of the cannibalistic salamander Hynobius retardatus. Behav Ecol Sociobiol 60:205–211
Michimae H, Wakahara M (2001) Factors which affect the occurrence of cannibalism and the broad-headed “cannibal” morph in larvae of the salamander Hynobius retardatus. Behav Ecol Sociobiol 50:339–345
Michimae H, Wakahara M (2002a) A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56:2029–2038
Michimae H, Wakahara M (2002b) Variation in cannibalistic polyphenism between populations in the salamander Hynobius retardatus. Zool Sci 19:703–707
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, New York
Nishihara A (1996) Effects of density on growth of head size in larvae of the salamander Hynobius retardatus. Copeia 1996:478–483
Ohdachi S (1994) Growth, metamorphosis, and gape-limited cannibalism and predation on tadpoles in larvae of salamanders, Hynobius retardatus. Zool Sci 11:127–131
Parichy DM, Kaplan RH (1995) Maternal investment and developmental plasticity: functional consequences for locomotor performance on hatchling frog larvae. Funct Ecol 9:606–617
Scott DE (1990) Effects of larval density in Ambystoma opacum: an experiment in large-scale field enclosures. Ecology 71:296–306
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396
Seymour RS, Bradford DF (1995) Respiration of amphibian eggs. Physiol Zool 68:1–25
Shaw RG, Byers DL (1998) Genetics of maternal and paternal effects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford, New York, pp. 97–111
Sinervo B, Doughty P (1996) Interactive effects of offspring size and timing of reproduction on offspring reproduction: experimental, maternal, and quantitative genetic aspects. Evolution 50:1314–1327
Thumm K, Mahony M (2005) Is variable egg size the proximate cause of diversified bet-hedging in the hatching dynamics of the red-crowned toadlet (Pseudophryne australis) (Anura: Myobatrachidae)? Herpetologica 61:9–19
Wade MJ (1998) The evolutionary genetics of maternal effects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford, New York, pp. 5–21
Wakahara M (1997) Kin recognition among intact and blinded, mixed-sibling larvae of a cannibalistic salamander Hynobius retardatus. Zool Sci 14:893–899
Acknowledgments
I thank Dr Masami Wakahara and two anonymous reviewers for their comments on the manuscript. This work was partly supported by a Grant-in-Aid for Scientific Research (No. 15009850) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Michimae, H. Differentiated egg size of the cannibalistic salamander Hynobius retardatus . J Ethol 25, 153–158 (2007). https://doi.org/10.1007/s10164-006-0009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-006-0009-9