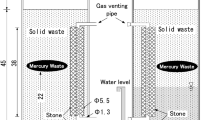
Abstract
The work is devoted to the study of a possible technology for the immobilization of mercury in waste, based on the principles of converting mercury and its compounds in waste into mercury sulfide. The purpose of this work is to develop an optimal method for immobilizing mercury in mercury-containing waste, and then compare the effectiveness of various stabilization methods. It is shown in the article that the frequently used immobilization of waste with sulfur may be ineffective and may lead, among other things, to the formation of ionic mercury. Therefore, the paper proposed to use pyrite instead of sulfur to immobilize mercury in waste. It is also shown that this process is more efficient and faster. Even better results are obtained using a mixture of pyrite and elemental sulfur. The analytical method of the mobile mercury and ionic mercury content in the final product estimation was developed. This analytical method is also used for determining mercury in solid samples. This final product is a powder mixture of glass, sulfur or pyrite, mercury sulfide, bentonite, and other mercury compounds including metallic mercury.







Similar content being viewed by others
References
Bower J, Savage KS, Weinman B, Barnett MO, Hamilton WP, Harper WF (2008) Immobilization of mercury by pyrite (FeS2). Environ Pollut 156(2):504–514. https://doi.org/10.1016/j.envpol.2008.01.011
Chalkidis A, Jampaiah D, Aryana A, Wood CD, Hartley PG, Sabri YM, Bhargava SK (2020) Mercury-bearing wastes: sources, policies and treatment technologies for mercury recovery and safe disposal. J Environ Manag 270:110945. https://doi.org/10.1016/j.jenvman.2020.110945
Chen CY, Driscoll CT, Eagles-Smith CA, Eckley CS, Gay DA, Hsu-Kim H, Keane SE, Kirk JL, Mason RP, Obrist D, Selin H, Selin NE, Thompson MR (2018) A critical time for mercury science to inform global policy. Environ Sci Technol 52(17):9556–9561. https://doi.org/10.1021/acs.est.8b02286
Drott A, Björn E, Bouchet S, Skyllberg U (2013) Refining thermodynamic constants for mercury (II)-sulfides in equilibrium with metacinnabar at sub-micromolar aqueous sulfide concentrations. Environ Sci Technol 47(9):4197–4203. https://doi.org/10.1021/es304824n
Makarova A, Meshalkin V, Fedoseev A, Kantyukov R, Kolybanov K (2020) Systems analysis of the efficiency of imitation processes of the chemical immobilization of mercury in waste using multivariant visualization tools. Theor Found Chem Eng 54:872–878. https://doi.org/10.1134/S0040579520050383
Fukuda N, Takaoka M, Oshita K, Mizuno T (2014) Stabilizing conditions of metal mercury in mercury sulfurization using a planetary ball mill. J Hazard Mater 276:433–441. https://doi.org/10.1016/j.jhazmat.2014.04.063
Gliniak M, Lis A, Los A, Mikołajek D, Kapłański Z (2020) Hazardous waste solidification from chemical technological process renewable energy sources: engineering, technology innovation. Springer, Cham, pp 727–734
Hamilton WP, Bowers AR (1997) Determination of acute Hg emissions from solidified/stabilized cement waste forms. Waste Manage 17(1):25–32. https://doi.org/10.1016/S0956-053X(97)00031-7
López FA, Alguacil FJ, Roman CP, Tayibi H, López-Delgado A (2008) Disposal of elemental mercury via sulphur reaction by milling. In: Abst 1st International Conference on “Hazardous Waste Management”. 1-3 Octubre, Chaina, Grete, Greece. p 476. https://digital.csic.es/bitstream/10261/7692/1/DISPOSAL%20ELEMENTALHg.pdf. Accessed 25 Apr 2021
Makarova AS, Yarovaya OV, Fedoseev AN, Yakubovich LM (2020) Development of a technology for immobilizing mercury in solid mercury-containing wastes. Clean Eng Technol. https://doi.org/10.1016/j.clet.2020.100030
Makarova A, Fedoseev A, Liubov Y (2019) Research on green technologies for immobilizing mercury in waste to minimize chemical footprint. Pure Appl Chem 92(4):557–565. https://doi.org/10.1515/pac-2019-0813
Murphy R, Strongin DR (2009) Surface reactivity of pyrite and related sulfides. Surf Sci Rep 64(1):1–45. https://doi.org/10.1016/j.surfrep.2008.09.002
Sjöblom R, Bjurström H, Pusch R (2003) Feasibility of compacted bentonite barriers in geological disposal of mercury-containing waste. Appl Clay Sci 23(1–4):8. https://doi.org/10.1016/S0169-1317(03)00102-9
Wang M, Li Y, Zhao D, Zhuang L, Yang G, Gong Y (2020) Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem Eng J 381:122664. https://doi.org/10.1016/j.cej.2019.122664
Yang Y, Chen T, Sumona M, Gupta BS, Sun Y, Hu Z, Zhan X (2017) Utilization of iron sulfides for wastewater treatment: a critical review. Rev Environ Sci Biotechnol 16:289–308. https://doi.org/10.1007/s11157-017-9432-3
Zhu Y, Peng S, Lu P, Chen T, Yang Y (2020) Mercury removal from aqueous solutions using modified pyrite: a column experiment. Minerals 10(1):43. https://doi.org/10.3390/min10010043
Acknowledgements
This research was funded by the Mendeleev University of Chemical Technology of Russia according 377 to research project # 3-2020-039.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anna, M., Andrey, F. & Eugenia, V. Comparison of the performance of different methods to stabilize mercury-containing waste. J Mater Cycles Waste Manag 24, 1134–1139 (2022). https://doi.org/10.1007/s10163-022-01386-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01386-w