Abstract
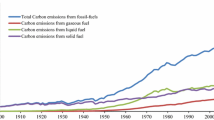
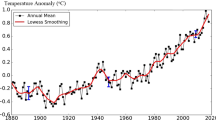
Increased energy consumption due to industrial growth has increased the levels of carbon dioxide (CO2) emission being released into the atmosphere. CO2 emission is a type of greenhouse gas which is a major cause of global warming. Since the issue of CO2 emissions has drawn much attention in recent years, the development of CO2 capture technology has become a necessity. Although CO2 adsorbents are still at the early development stage, it has been suggested that CO2 adsorbents are the most effective technology in controlling CO2 emissions. Solid adsorbents have great potential as an alternative method to conventional adsorbents in adsorbing CO2. In this paper, low cost adsorbents including activated carbon, zeolites, mesoporous silica and clays are discussed in terms of adsorbent preparation methods and CO2 adsorption capacity. The low cost adsorbents are mainly derived from waste materials such as fly ash, steel slag, red mud, bagasses wastes and wood wastes. Besides that, natural resources such as clays have also been applied as low cost CO2 adsorbents. Surface modifications have also been applied to the low cost adsorbents, including metal ion exchange and amine impregnation to enhance CO2 adsorption capacity. In the last section, the current status of CO2 adsorbents is summarized and future trends are discussed briefly to predict the potential materials which can be applied as CO2 adsorbents.

Similar content being viewed by others
References
Oh TH (2010) Carbon capture and storage potential in coal-fired plant in Malaysia—a review. Renew Sustain Energy Rev 14:2697–2709. doi:10.1016/jrser.2010.06.003
Yang AL, Cui YY (2012) Global coal risk assessment: data analysis and market research. WRI Working Paper. World Resources Institute. http://www.wri.org/publication/global-coal-risk-assessment. Accessed 28 Aug 2014
Othman MR, Martunus Zakaria R, Fernando WJN (2009) Strategic planning on carbon capture from coal fired plants in Malaysia and Indonesia: a review. Energy Policy 37:1718–1735. doi:10.1016/j.enpol.2008.12.034
Postdam Institute (2012) Turn down the heat: why a 4 °C warmer world must be avoided. Georgetown Law. Georgetown climate center. The World Bank. http://www.georgetownclimate.org/resources/turn-down-the-heat-why-a-4-c-warmer-world-must-be-avoided. Accessed 28 Aug 2014
Lu CS, Bai HL, Wu B, Su FS, Hwang JF (2008) Comparative study of CO2 capture by carbon nanotubes, activated carbons and zeolites. Energy Fuels 22:3050–3056. doi:10.1021/ef8000086
Alessandro DMD, Smit B, Long JR (2010) Carbon dioxide capture: prospects for New Materials. Angew Chem Int Ed 49:6058–6082. doi:10.1002/anie.201000431
Majchrzak-Kucęba I, Nowak W (2010) Development of fly ash-based sorbent to capture CO2 from flue gas. In: Proceedings of the 20th international conference on fluidized bed combustion 596–602. doi:10.1007/978-3-642-02682-9_90
Chou CT, Chen FH, Huang YJ, Yang HS (2013) Carbon dioxide capture and hydrogen purification from synthesis gas by pressure swing adsorption. Chem Eng Trans 32:1855–1860. doi:10.3303/CET1332310
Hauchhum L, Mahanta P (2014) Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int J Energy Environ Eng 5:349–356. doi:10.1007/s40095-014-0131-3
Aspelund A (2010) Gas purification, compression and liquefaction processes and technology for carbon dioxide (CO2) transport. In: Maroto-Valer MM (ed) Development and innovation in carbon dioxide (CO-2) capture and storage technology, vol 1., Carbon dioxide (CO2) capture, transport and industrial applicationsWoodhead Publishing Ltd, Cambridge, pp 383–407
Bouzalakos S, Maroto-Valer MM (2010) Overview of carbon dioxide (CO2) capture and storage technology. In: Maroto-Valer MM (ed) Development and innovation in carbon dioxide (CO2) capture and storage technology, vol 2., Carbon dioxide (CO2) storage and utilisationWoodhead Publishing Ltd, Cambridge, pp 1–24
Lee KM, Jo YM (2010) Synthesis of zeolite from waste fly ash for adsorption of CO2. J Mater Cycles Waste Manage 12:212–219. doi:10.1007/s10163-010-0290-0
Liu LY, Singh R, Xiao Penny, Webley PA, Zhai YC (2011) Zeolite synthesis from waste fly ash and its application in CO2 capture from flue gas streams. Adsorption 17:795–800. doi:10.1007/s10450-011-9332-8
Boonpoke A, Chiarakorn S, Laosiripojana N, Towprayoon S, Chidthaisong A (2012) Investigation of CO2 adsorption by bagasse-based activated carbon. Korean J Chem Eng 29:89–94. doi:10.1007/s11814-011-0143-0
Ghani WAMAK, Mohd A, da Silva G, Bachmann RT, Taufiq-Yap YH, Rashid U, Al-Muhtaseb AH (2013) Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: chemical and physical characterization. Ind Crops Prod 44:18–24. doi:10.1016/j.indcrop.2012.10.017
Somy A, Mehrnia MR, Amrei HD, Ghanizadeh A, Safari M (2009) Adsorption of carbon dioxide using impregnated activated carbon promoted by zinc. Int J Greenh Gas Control 3:249–254. doi:10.1016/j.ijggc.2008.10.003
Yang R, Liu GQ, Li M, Zhang JC, Hao XM (2012) Preparation and N2, CO2 and H2 adsorption of super activated carbon derived from biomass source hemp (Cannabis sativa L.) stem. Microporous Mesoporous Mater 158:108–116. doi:10.1016/j.micromeso.2012.03.004
Chen ZH, Deng SD, Wei HR, Wang B, Huang J, Yu G (2013) Activated carbons and amine-modified materials for carbon dioxide capture—a review. Front Environ Sci Eng 7:326–340. doi:10.1007/s11783-013-0510-7
Witoon T (2012) Polyethyleneimine-loaded bimodal porous silica as low-cost and high-capacity sorbent for CO2 capture. Mater Chem Phys 137:235–245. doi:10.1016/j.matchemphys.2012.09.014
Liu Y, Guo YP, Gao W, Wang Z, Ma YJ, Wang ZC (2012) Simultaneous preparation of silica and activated carbon from rice husk ash. J Cleaner Prod 32:204–209. doi:10.1016/j.jclepro.2012.03.021
Olivares-Marin M, Maroto-Valer M (2012) Development of adsorbents for CO2 capture from waste materials: a review. Greenhouse Gas Sci Technol 2:20–35. doi:10.1002/ghg.45
Balakrishnan B, Abdul Awal ASM (2014) Durability properties of concrete containing high volume Malaysian fly ash. Int J Res Eng Technol 3:529–533
Chindaprasirt P, Jaturapitakkul C, Sinsiri T (2005) Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem Concr Compos 27:425–428. doi:10.1016/j.cemconcomp.2004.07.003
Chaipanich A, Nochaiya T, Wongkeo W, Torkittikul P (2010) Compressive strength and microstructure of carbon nanotubes-fly ash cement composites. Mater Sci Eng A 527:1063–1067. doi:10.1016/j.msea.2009.09.039
Goñi S, Fridas M, Vegas I, García R, Vigil de Villa R (2012) Effect of ternary cements containing thermally activated paper sludge and fly ash on the texture of C–S–H gel. Constr Build Mater 30:381–388. doi:10.1016/j.conbuildmat.2011.12.002
NurIzaati MN, Shamsul JB, Mazlee MN (2012) Development and properties of coconut fiber reinforced composite cement with the addition of fly ash. J Sustain Cement based Mater 1:186–191. doi:10.1080/21650373.2012.754569
Zeng Q, Li KF, Fen-Chong T, Dangla P (2012) Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr Build Mater 27:560–569. doi:10.1016/j.conbuildmat.2011.07.007
Roshasmawi AW, Mazlee MN, Shamsul JB, Khairul Nizar T (2013) Effects of fly ash addition on compressive strength and flexural strength of foamed cement composites. Adv Mater Res 795:664–668. doi:10.4028/www.scientific.net/AMR.795.664
Mustafa Al Bakri AM, Kamarudin H, Bnhussain M, Khairul Nizar I, Rafiza AR, Zarina Y (2012) The processing, characterization and properties of fly ash based geopolymer concrete. Rev Adv Mater Sci 30:90–97
Cicek T, Tannverdi M (2007) Lime based steam autoclaved fly ash bricks. Constr Build Mater 21:1295–1300. doi:10.1016/j.conbuildmat.2006.01.005
Cultrone G, Sebastían E (2009) Fly ash addition in clayey materials to improve the quality of solid bricks. Constr Build Mater 23:1178–1184. doi:10.1016/j.conbuild.mat.2008.07.001
Akhtar JN, Alam J, Akhtar MN (2010) An experimental study on fibre reinforced fly ash based lime bricks. Int J Phys Sci 5:1688–1695
Kumar R, Kulkami AD, Attar SJ, Kulkami KS (2011) Utilization of fly ash for bricks manufacturing. Int J Adv Eng Tech 2:359–362
Xu L, Guo W, Wang T, Yang N (2005) Study on fired bricks with replacing clay by fly ash in high volume ratio. Constr Build Mater 19:243–247. doi:10.1016/j.conbuildmat.2004.05.017
Arenillas A, Smith KM, Drage TC, Snape CE (2005) CO2 capture using some fly ash-derived carbon materials. Fuel 84:2204–2210. doi:10.1016/j.fuel.2005.04.003
Kaithwas A, Prasad M, Kulshrestha A, Verma S (2012) Industrial wastes derived solid adsorbents for CO2 capture: a mini review. Chem Eng Res Des 90:1632–1641. doi:10.1016/j.cherd.2012.02.011
Maroto-Valer MM, Lu Z, Zhang YZ, Tang Z (2008) Sorbents for CO2 capture from high carbon fly ashes. Waste Manage 28:2320–2328. doi:10.1016/j.wasman.2007.10.012
Park JE, Youn HK, Yang ST, Ahn WS (2012) CO2 capture and MWCNTs synthesis using mesoporous silica and zeolite 13X collectively prepared from bottom ash. Catal Today 190:15–22. doi:10.1016/j.cattod.2011.09.032
Majchrzak-Kućeba I, Nowak W (2005) A thermogravimetric study of the adsorption of CO2 on zeolites synthesized from fly ash. Thermochim Acta 437:67–74. doi:10.1016/j.tca.2005.06.003
Yaumi AL, Hussein IA, Shawabkeh RA (2013) Surface modification of oil fly ash and its application in selective capturing of carbon dioxide. Appl Surf Sci 266:118–125. doi:10.1016/j.apsusc.2012.11.109
Davini P (2002) Flue gas treatment by activated carbon obtained from oil-fired fly ash. Carbon 40:1973–1979. doi:10.1016/S0008-6223(02)00049-0
Oh GH, Yun CH, Park CR (2003) Role of KOH in one-stage KOH activation of cellulosic biomass. Carbon Sci 4:1–11. doi:10.1016/j.apsusc.2012.11.109
Bankole LK, Rezan SA, Sharif NM (2014) Assessment of hexavalent chromium release in Malaysian electric arc furnace steel slag for fertilizer usage. Earth Environ Sci 19:1–7. doi:10.1088/1755-1315/19/1/012004
Reddy AS, Pradhan RK, Chandra S (2006) Utilization of basic oxygen (BOF) slag in the production of a hydraulic cement binder. Int J Miner Process 79:98–105. doi:10.1016/j.minpro.2006.01.001
Bankole LK, Rezan SA, Sharif NM, Baharun N (2013) Evaluation of leaching behaviour of hexavalent chromium from Malaysian electric arc furnace steel slag. Adv Mater Res 652–654:1628–1632. doi:10.4028/www.scientific.net/AMR.652-654.1628
Bonenfant D, Kharoune L, Sauve S, Hausler R, Niquette P, Mimeault M, Kharoune M (2008) CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind Eng Chem Res 47:7610–7616. doi:10.1021/ie701721j
Bonenfant D, Kharoune L, Sauve S, Hausler R, Niquette P, Mimeault M, Kharoune M (2009) Molecular analysis of carbon dioxide adsorption process on steel slag oxides. Int J Greenhouse Gas Control 3:20–28. doi:10.1016/j.ijggc.2008.06.001
Yi H, Xu GP, Cheng HG, Wang JS, Wan YF, Chen H (2012) An overview of utilization of steel slag. Procedia Environ Sci 16:791–801. doi:10.1016/j.proenv.2012.10.108
Proctor DM, Fehling KA, Shay EC, Wittenborn JL, Green JJ, Avent C, Bigham RD, Connolly M, Lee B, Shepker TO, Zak MA (2000) Physical and chemical characteristics of blast furnace, basic oxygen furnace and electric arc furnace steel industry slags. Environ Sci Technol 34:1576–1582. doi:10.1021/es9906002
Doucet FJ (2010) Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner Eng 23:262–269. doi:10.1016/j.mineng.2009.09.006
Sun RY, Li YJ, Zhao JL, Liu CT, Lu CM (2013) CO2 capture using carbide slag modified by propionic acid in calcium looping process for hydrogen production. Int J Hydrog Energy 38:13655–13663. doi:10.1016/j.ijhydene.2013.08.030
Pan SY, Chang EE, Chiang PC (2012) CO2 capture by accelerated carbonation of alkaline waste: a review on its principle and applications. Aerosol Air Qual Res 12:770–791. doi:10.4209/aaqr.2012.06.0149
Kunzler C, Alves N, Pereira E, Nienzewksi J, Ligabue R, Einloft S (2011) CO2 storage with indirect carbonation using industrial waste. Energy Procedia 4:1010–1017. doi:10.1016/j.egypro.2011.01.149
Huijgen WJJ, Witkamp GJ, Comans RNJ (2005) Mineral CO2 sequestration by steel slag carbonation. Environ Sci Technol 39:9676–9682. doi:10.1021/es050795f
Yu J, Wang K (2011) Study on characteristic of steel slag for CO2 capture. Energy Fuels 25:5483–5492. doi:10.1021/ef2004255
Bonenfant D, Kharoune L, Sauvé S, Hausler R, Niquette P, Mimeault M, Kharoune M (2008) CO2 sequestration by aqueous red mud carbonation at ambient pressure and temperature. Ind Eng Chem Res 47:7617–7622. doi:10.1021/ie7017228
Jones G, Joshi G, Clark M, McConchie D (2006) Carbon capture and the aluminium industry: preliminary studies. Environ Chem 3:297–303. doi:10.1071/EN06018
Yadav VS, Prasad M, Khan J, Amritphale SS, Singh M, Raju CB (2010) Sequestration of carbon dioxide (CO2) using red mud. J Hazard Mater 176:1044–1050. doi:10.1016/j.jhazmat.2009.11.146
Johnston M, Clark MW, McMahon P, Ward N (2010) Alkalinity conversion of bauxite refinery residues by neutralization. J Hazard Mater 182:710–715. doi:10.1016/j.jhazmat.2010.06.091
Lin LY, Bai HL (2012) Aerosol processing of low-cost mesoporous silica spherical particles from photonic industrial waste powder for CO2 capture. Chem Eng J 197:215–222. doi:10.1016/j.cej.2012.04.103
Shafie SM, Mahlia TMI, Masjuki HH, Ahmad-Yazid A (2012) A review on electricity generation based on biomass residue in Malaysia. Renew Sustain Energy Rev 16:5879–5889. doi:10.1016/j.rser.2012.06.031
Savova D, Apak E, Ekinci E, Yardim F, Petrov N, Budinova T, Razvigorova M, Minkova V (2001) Biomass conversion to carbon adsorbents and gas. Biomass Energy 21:133–142. doi:10.1016/S0961-9534(01)00027-7
Foo KY, Hameed BH (2009) Utilization of rice husk ash as novel adsorbent: a judicious recycling of the colloidal agricultural waste. Adv Colloid Interface Sci 152:39–47. doi:10.1016/j.cis.2009.09.005
Plaza MG, Pevida C, Arias B, Fermoso J, Casal MD, Martin CF, Rubiera F, Pis JJ (2009) Development of low-cost biomass-based adsorbents for post combustion CO2 capture. Fuel 88:2442–2447. doi:10.1016/j.fuel.2009.02.025
Idrisa MN, Zainal AA, Ahmad MA (2011) Adsorption equilibrium of malachite green dye onto rubber seed coat based activated carbon. Int J Basic Appl Sci 11:305–311
Bandosz TJ, Ania CO (2006) Surface chemistry of activated carbons and its characterization. In: Bandosz TJ (ed) Activated carbon surface environmental remediation. Elsevier, Amsterdam, pp 159–230
Plaza MG, Gracía S, Rubiera F, Pis JJ, Pevida C (2011) Evaluation of ammonia modified and conventionally activated biomass based carbons as CO2 adsorbents in post combustion condition. Sep Purif Technol 80:96–104. doi:10.1016/j.seppur.2011.04.015
Plaza MG, Rubiera F, Pis JJ, Pevida C (2010) Ammoxidation of carbon materials for CO2 capture. Appl Surf Sci 256:6843–6849. doi:10.1016/j.apsusc.2010.04.099
González AS, Plaza MG, Rubiera F, Pevida C (2013) Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem Eng J 230:456–465. doi:10.1016/j.cej.2013.06.118
Nasri NS, Hamza UD, Ismail SN, Ahmed MM, Mohsin R (2014) Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J Clean Prod 71:148–157. doi:10.1016/j.jclepro.2013.11.053
Foo KY, Hameed BH (2013) Textural porosity, surface chemistry and adsorptive properties of durian shell derived activated carbon prepared by microwave assisted NaOH activation. Chem Eng J 187:53–62. doi:10.1016/j.cej.2012.01.079
Rashidi NA, Yusup S, Hameed BH (2013) Kinetic studies on carbon dioxide capture using lignocellulosic based activated carbon. Energy 61:440–446. doi:10.1016/j.energy.2013.08.050
Boonpoke A, Chiarakon S, Laosiripojana N, Towprayoon S, Chidthaisong A (2011) Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J Sustain Energy Environ 2:77–81
Maroto Valer MM, Tang Z, Zhang Y (2005) CO2 capture by activated and impregnated anthracites. Fuel Process Technol 86:1487–1502. doi:10.1016/j.fuproc.2005.01.003
Jang HT, Park YK, Ko YS, Lee JY, Margandan B (2009) Highly siliceous MCM-48 from rice husk ash for CO2 adsorption. Int J Greenh Gas Control 3:545–549. doi:10.1016/j.ijggc.2009.02.008
Kim S, Ida J, Guliants VV, Lin YS (2005) Tailoring pore properties of MCM-48 silica for selective adsorption of CO2. J Phys Chem B 109:6287–6293. doi:10.1021/jp045634x
Chandrasekar G, Son WJ, Ahn WS (2008) Synthesis of mesoporous materials SBA-15 and CMK-13 from fly ash and their application for CO2 adsorption. J Porous Mater 16:545–551. doi:10.1007/s10934-008-9231-x
Margandan B, Lee JY, Ramani A, Jang HT (2010) Utilization of rice husk ash as silica source for the synthesis of mesoporous silicas and their application to CO2 adsorption through TREN/TEPA grafting. J Hazard Mater 175:928–938. doi:10.1016/j.hazmat.2009.10.097
Heidari A, Younesi H, Rashidi A, Ghoreyshi A (2014) Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: effect of chemical activation. J Taiwan Inst Chem Eng 45:579–588. doi:10.1016/j.jtice.2013.06.007
Heidari A, Younesi H, Rashidi A, Ghoreyshi A (2014) Evaluation of CO2 with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem Eng J 254:503–513. doi:10.1016/j.cej.2014.06.004
Zhu XL, Wang PY, Peng C, Yang J, Yan XB (2014) Activated carbon produced from paulownias sawdust for high-performance CO2 sorbents. Chin Chem Lett 25:929–932. doi:10.1016/j.cclet.2014.03.039
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179. doi:10.1016/j.cej.2014.03.105
Kannan S, Bakar MZA (2004) Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 27:89–96. doi:10.1016/j.biombioe.2003.11.002
Patnukao P, Pavasant P (2008) Activated carbon from Eucalyptus camaldulensis Dehn bark using phosphoric acid activation. Bioresour Technol 99:8540–8543. doi:10.1016/j.biortech.2006.10.049
Houshmand A, Daud WMAW, Shafeeyan MS (2011) Exploring potential methods for anchoring amine groups on the surface of activated carbon for CO2 adsorption. Sep Sci Technol 46:1098–1112. doi:10.1080/01496395.2010.546383
Drisko GL, Aquino C, Feron PHM, Caruso RA, Harrisson S, Luca V (2013) One-pot preparation and CO2 adsorption modelling of porous carbon, metal oxide and hybrid beads. ACS Appl Mater Interface 5:5009–5014. doi:10.1021/am4007929
Timur S, Kantarli IC, Onenc S, Yanik J (2010) Characterization of application of activated carbon produced from oak cup pulp. J Anal Appl Pyrolysis 89:129–136. doi:10.1016/j.jaap.2010.07.002
Pevida C, Arias B, Fermoso J, Rubiera F, Pis JJ (2008) Surface modification of activated carbon for CO2 capture. Appl Surf Sci 254:7165–7172. doi:10.1016/j.apsusc.2008.05.239
Cheng F, Liang J, Zhao J, Tao Z, Chen J (2008) Biomass waste-derived microporous carbon with controlled texture and enhanced hydrogen uptake. Chem Mater 20:1889–1895. doi:10.1021/cm702816x
Guo Y, Rockstraw DA (2007) Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour Technol 98:1513–1521. doi:10.1016/j.biortech.2006.06.027
Zhang CM, Song W, Sun GH, Xie LJ, Wang JL, Li KX, Sun CG, Liu H, Snape CE, Drage T (2013) CO2 capture with activated carbon grafted by nitrogenous functional groups. Energy Fuels 27:4818–4823. doi:10.1021/ef400499k
Delgado JA, Uguina MA, Sotelo JL, Ruíz B, Rosário M (2007) Carbon dioxide/methane separation by adsorption on sepiolite. J Nat Gas Chem 16:235–243. doi:10.1016/S1003-9953(07)60054-1
Pires J, Bestilleiro M, Pinto M, Gil A (2008) Selective adsorption of carbon dioxide, methane and ethane by porous clays heterostructures. Sep Purif Technol 61:161–167. doi:10.1016/j.seppur.2007.10.007
Azzouz A, Platon N, Nousir S, Ghomari K, Nistor D, Shiao TC, Roy R (2013) OH-enriched organo-montmorillonites for potential application in carbon dioxide separation and concentration. Sep Purif Technol 108:181–188. doi:10.1016/j.seppur.2013.02.006
Chen C, Park DW, Ahn WS (2013) Surface modification of a low cost bentonite for post-combustion CO2 capture. Appl Surf Sci 283:699–704. doi:10.1016/j.apsusc.2013.07.005
Azzouz A, Nousir S, Platon N, Ghomari K, Shiao TC, Hersant G, Bergeron JY, Roy R (2013) Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int J Greenh Gas Control 17:140–147. doi:10.1016/j.ijggc.2013.04.013
Wang WL, Xiao J, Wei XL, Wang XX, Song CS (2014) Development of a new clay supported polyethyleneimine composite for CO2 capture. Appl Energy 113:334–341. doi:10.1016/j.apemergy.2013.03.090
Reijers HTJ, Valster-Schiermeier SEA, Cobden PD, Brink RVD (2006) Hydrotalcite as CO2 sorbent for sorption-enhanced steam reforming of methane. Ind Eng Chem Res 45:2522–2530. doi:10.1021/ie050563p
Gil A, Trujillano R, Vicente MA, Korili SA (2007) Adsorption of nitrogen, hydrogen and carbon dioxide on alumina-pillared clays. Stud Surf Sci Catal 160:327–334. doi:10.1016/S0167-2991(07)80043-7
Pawar RR, Patel HA, Sethia G, Bajaj HC (2009) Selective adsorption of carbon dioxide over nitrogen on calcined synthetic hectorites with tailor-made porosity. Appl Clay Sci 46:109–113. doi:10.1016/j.clay.2009.07.009
Stevens L, Williams K, Wong YH, Drage T, Snape C, Wood J, Wang JW (2013) Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem Eng J 215–216:699–708. doi:10.1016/j.cej.2012.11.058
Nousir S, Platon N, Ghomari K, Sergentu AS, Shiao TC, Hersant G, Bergeron JY, Roy R, Azzouz A (2013) Correlation between the hydrophilic character and affinity towards carbon dioxide of montmorillonite-supported polyalcohols. J Colloid Interface Sci 402:215–222. doi:10.1016/j.jcis.2013.03.050
Wang WL, Wang XX, Song CS, Wei XL, Ding J, Xiao J (2013) Sulphuric acid modified bentonite as the support of tetraethylenepentamine for CO2 capture. Energy Fuels 27:1538–1546. doi:10.1021/ef3021816
Di Cosimo JI, Diez VK, Xu M, Iglesia E, Apesteguia CR (1998) Structure and surface and catalytic properties of Mg-Al based basic oxides. J Catal 178:499–510. doi:10.1006/jcat.1998.2161
Gil A, Gandía LM, Vicente MA (2000) Recent advances in the synthesis and catalytic applications of pillared clays. Catal Rev Sci Eng 42:145–212. doi:10.1081/CR-100100261
Gil A, Gandía LM (2003) Microstructure and quantitative estimation of the micropore-size distribution of an alumina-pillared clay from nitrogen adsorption at 77LK and carbon dioxide adsorption at 273K. Chem Eng Sci 58:3059–3075. doi:10.1016/S0009-2509(03)00182-9
Jaroniec M, Gadkaree KP, Choma J (1996) Relation between adsorption potential distribution and pore volume distribution for micropores carbons. Colloids Surf A 118:203–210. doi:10.1016/S0927-7757(96)03685-0
Sircar S (2006) Basic research need for design of adsorptive gas separation process. Ind Eng Chem Res 45:5435–5448. doi:10.1021/ie051056a
Azzouz A, Messad D, Nistor D, Catrinescu C, Zvolinschi A, Asaftei S (2003) Vapour phase aldol condensation over fully ion-exchanged montmorillonite-rich catalysts. Appl Catal A Gen 24:1–13. doi:10.1016/S0926-860X(02)00524-0
Xu XC, Song CS, Andresen JM, Miller BG, Scaroni AW (2002) Novel polyethylenimide-modified mesoporous molecular sieve of MCM-41 type as high capacity adsorbent for CO2 capture. Energy Fuel 16:1463–1469. doi:10.1021/ef02005
Qi G, Wang Y, Estevez L, Duan X, Anako N, Park AHA, Li W, Jones CW, Giannelis EP (2011) High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ Sci 4:444–452. doi:10.1039/C0EE00213E
Acknowledgments
The authors wish to thank the Ministry of Higher Education of Malaysia (Grant no.: 9003-00412) for financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, W.H., Mazlee, M.N., Ahmad, Z.A. et al. The development of low cost adsorbents from clay and waste materials: a review. J Mater Cycles Waste Manag 19, 1–14 (2017). https://doi.org/10.1007/s10163-015-0396-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-015-0396-5