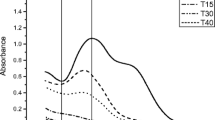
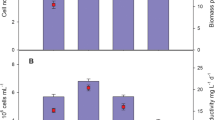
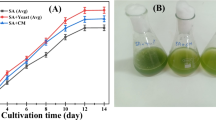
Abstract
Livestock wastewater is treated by activated sludge treatment. Untreated livestock wastewater has high estrogen activity because animal excreta contains estrogen. When activated sludge treatment is applied, the estrogen activity declines or is lost. However, the color of treated livestock wastewater is deep brownish-red because of the decomposition of organic compounds or the synthesis of metabolites. Discharging colored wastewater to the environment could cause some problems, so it is necessary to decolorize colored wastewater before it is discharged. It has been suggested that electrolysis decolorization technology is suitable for treating colored wastewater; however, the process produces volatile organic compounds (VOCs). In fact, little research has been conducted with reference to estrogen activity in wastewater that has undergone electrolysis, especially on the contribution of the electrolysis decolorization process to estrogen activity, i.e., the possibility of resynthesis of some substance with estrogen activity due to resolved and metabolized colored components. In this study, the concentration of VOC was measured for various electrolysis conditions, and estrogen activity was examined using a yeast two-hybrid assay. From the results, decolorization of colored livestock wastewater by electrolysis was possible, and the VOC generation during electrolysis could be controlled depending on the electrolysis conditions. Estrogen activity in colored livestock wastewater disappeared on electrolysis decolorization.
Similar content being viewed by others
References
Mori T, Sakimoto M, Sakai T (1997) Decolorization of wastewater from a livestock barn using andosols. Anim Sci Technol 68:940–947
Joaquim CA, Esteves DS, Machado A, Silva M (1998) Acid-base properties of fulvic acids extracted from an untreated sewage sludge and from composted sludge. Wat Res 32:441–449
Maillard LC (1912) Action of amino acid on sugars. Formation of melanoidins in a methodical way. Comp Rend 154:66–68
Hayase F (1987) Chemistry of melanoidians (in Japanese). Nippon Nogeikagaku Kaishi 61:970–973
Hayase F, Kim SB, Kato H (1986) Analyses of the chemical structures of melanoidins by 13C NMR, 13C and 15N CP-MAS NMR spectrometry. Agric Biol Chem 50:1951–1957
Kim SB, Hayase F, Kato H (1985) Decolorization and degradation products of melanoidins on ozonolysis. Agric Biol Chem 49:785–792
Hong SW, Choi YS, Kwon G, Park KY (2005) Performance evaluation of physicochemical processes for biologically pre-treated livestock wastewater. Water Sci Technol 52:107–115
Martin CA, Alfona OM, Cassano AE (2001) Water decolorization using UV radiation and hydrogen peroxide. Water Sci Technol 44:53–60
Martin CA, Alfona, OM, Cassano AE (2000) Decolorization of water for domestic supply employing UV radiation and hydrogen peroxide. Catalysis Today 60:119–127
Lee S-H, Iamchaturapatr J, Polprasert C, Ahn K-H (2004) Application of chemical precipitation for piggery wastewater treatment. Water Sci Technol 49:381–388
Dychdala GR (1991) Chlorine and chlorine compounds. Disinfection, sterilization, and preservation, 4th edn. pp 131–151
Kohno S, Kawata T, Kaku M, Fujita T, Tsutsui K, Ohtani J, Tenjo K, Motokawa M, Tohma Y, Shigekawa M, Kamata H, Tanne K (2004) Bactericidal effect of acidic electrolyzed water on the dental unit waterline. Jpn J Infect Dis 57:52–54
Nakajima N, Nakano T, Harada F, Taniguchi H, Yokoyama I, Hirose J, Daikoku E, Sano K (2004) Evaluation of disinfective potential of reactive free chlorine in pool tap water by electrolysis. J Micro Methods 57:163–173
Sharma RS, Demirci A (2003) Treatment of Escherichia coli O157:H7 inoculated alfalfa seeds and sprouts with electrolyzed oxidizing water. Int J Food Micro 86:231–237
Russell SM (2003) The effect of electrolyzed oxidative water applied using electrostatic spraying on pathogenic and indicator bacteria on the surface of eggs. Poult Sci 82:158–162
Takenouchi T, Yoshiike J, Wakabayashi S (2003) Evaluation for surface cleanness of metals washed by the electrolyzed alkaline water. J Surf Finish Soc Jpn 54(11):818–822
Takenouchi T, Yoshiike J, Wakabayashi S (2004) Evaluation for surface cleanness of metals washed by the electrolyzed acid water. J Surf Finish Soc Jpn 55(3):208–213
Callahan MA, Slimak MW, Gabel NW (1979) Water-related environmental fate of 129 priority pollutants, vol 1. EPA-440/4 79-029a, US Environmental Protection Agency, Washington, DC
Andersen H, Siegrist H, Halling B, Ternes TA (2003) Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37(18):4021–4026
Hashimoto T, Onda K, Nakamura Y, Tada K, Miya A, Mishina F (2004) Fate and behavior of endocrine disrupters in wastewater treatment plants. J Jpn Soc Wat Environ 27(12):797–802
Holbrook RD, Novak JT, Grizzard TJ, Love NG (2002) Estrogen receptor agonist fate during wastewater and biosolids treatment processes. Environ Sci Technol 36(21):4533–4539
Shimazu T, Wanami K, Yanagida H, Tamura K (2002) Study on endocrine disrupters in Tokyo’s rivers (11) income and outcome of estrogen in sewage disposal plants. Annual Report of the Tokyo Metropolitan Research Institute for Environmental Protection, Tokyo, pp 75–83
Soto AM, Calabro JM, Prechtl NV, Yau AY, Orlando EF, Daxenberger A, Kolok AS, Guillette LJ Jr, le Bizec B, Lange IG, Sonnenschein C (2004) Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in Eastern Nebraska, USA. Environ Health Perspect 112(3):346–352
Shiraishi F, Shiraishi H, Nishikawa J, Nishihara T, Morita M (2000) Development of a simple operational estrogenicity assay system using the yeast two-hybrid system. J Environ Chem 10(1):57–64
Nakagawara S, Goto T, Nara M, Ozawa Y, Hotta K, Arata Y (1998) Spectroscopic characterization and the pH dependence of bactericidal activity of aqueous chlorine solution. Anal Sci 14:691–698
Nagano A, Nakamoto C, Suzuki M (2000) Decolorization treatment by electrolysis of wastewater: characteristics of colored components and effects of electrodes. J Wat Environ Technol 23:34–40
Nagano A, Suzuki M (2001) Decolorization treatment by electrolysis of wastewater: effects of materials of electrodes on decolorization efficiency and pilot-scale experiment for practical use. J Wat Environ Technol 24:382–388
Takayama K, Takahashi N, Sakurai T, Tuzimoto K, Yoshimura T (2006) Development of an electrochemical and microbial degradation system for textile wastewater. J Technol Edu 13:63–70
Diamadoulous E, Sakellariadis D, Koukouraki E (1998) The effect of humic acid on the transport of volatile chlorinated hydrocarbons in soil. Wat Res 32(11):3325–3330
Lee J-W, Choi D-Y, Kwak D-H, Jung H-J (2005) Adsorption dynamics of water vapor on activated carbon. Adsorption 11:437–441
Huang J, Takatori K, Kumagai S, Takahashi J (1997) Fungicidal effect of oxidizing potential liquid. J Antibact Antifung Agents 25(7):387–391
Iwasawa A, Nakamura Y (1999) Antibacterial activity and safety of the strong acidity electrolysis water. J Antibact Antifung Agents 27(7):449–462
Iwasawa A, Nakamura Y, Shigeyama K, Tanba T, Nishimoto Y (2002) Comparison of methods for measuring available chlorine in strong acidic electrolyzed water. J Antibact Antifung Agents 30(10):627–634
Iwasawa A, Nakamura Y, Niwa T, Nishimoto Y (2002) The influence of pH on bactericidal effects of strong acidic electrolyzed water. J Antibact Antifung Agents 30(10):635–644
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kai, H., Ishibashi, Y., Mori, T. et al. Decolorization and estrogenic activity of colored livestock wastewater after electrolysis treatment. J Mater Cycles Waste Manag 12, 128–135 (2010). https://doi.org/10.1007/s10163-009-0273-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-009-0273-1