Abstract
Because cochlear implants function by stimulating the auditory nerve, it is assumed that the condition of the nerve plays an important role in the efficacy of the prosthesis. Thus, considerable research has been devoted to methods of preserving the nerve following deafness. Neurotrophins have been identified as a potential contributor to neural health, but most of the research to date has been done in young animals and for short periods (less than 3 to 6 months) after the onset of treatment. The first objective of the current experiment was to examine the effects of a neurotrophin gene therapy delivery method on spiral ganglion neuron (SGN) preservation and function in the long term (5 to 14 months) in mature guinea pigs with cochlear implants. The second objective was to examine several potential non-invasive monitors of auditory nerve health following the neurotrophin gene therapy procedure. Eighteen mature adult male guinea pigs were deafened by cochlear perfusion of neomycin and then one ear was inoculated with an adeno-associated viral vector with an Nft3-gene insert (AAV.Ntf3) and implanted with a cochlear implant electrode array. Five control animals were deafened and inoculated with an empty AAV and implanted. Data from 43 other guinea pig ears from this and previous experiments were used for comparison: 24 animals implanted in a hearing ear, nine animals deafened and implanted with no inoculation, and ten normal-hearing non-implanted ears. After 4 to 21 months of psychophysical and electrophysiological testing, the animals were prepared for histological examination of SGN densities and inner hair cell (IHC) survival. Seventy-eight percent of the ears deafened and inoculated with AAV.Ntf3 showed better SGN survival than the 14 deafened-control ears. The degree of SGN preservation following the gene therapy procedure was variable across animals and across cochlear turns. Slopes of psychophysical multipulse integration (MPI) functions were predictive of SGN density, but only in animals with preserved IHCs. MPI was not affected by the AAV.Ntf3 treatment, but there was a minor improvement in temporal integration (TI). AAV.Ntf3 treatment had significant effects on ECAP and EABR amplitude growth func-tion (AGF) slopes; the reduction in slope in deafened ears was ameliorated by the AAV.Ntf3 treatment. Slopes of the ECAP and EABR AGFs were predictive of SGN density in a broad area near and just apical to the implant. The highest ensemble spontaneous activity (ESA) values were seen in animals with surviving IHCs, but AAV.Ntf3 treatment in deafened ears resulted in slightly higher ESA values compared to deafened untreated ears. Overall, a combination of the psychophysical and electrophysiological measures can be useful for monitoring the health of the implanted cochlea in guinea pigs. These measures should be applicable for assessing cochlear health in human subjects.









Similar content being viewed by others
References
Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Hume CR, O'Leary SJ, Shepherd RK, Richardson RT (2012) Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS One 7:e52338
Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT (2014) Viability of long-term gene therapy in the cochlea. Sci Rep 4:4733
Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J (2006) Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 14:564–570
Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y (2015) Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep 5:8619
Dolan DF, Nuttall AL, Avinash G (1990) Asynchronous neural activity recorded from the round window. J Acoust Soc Am 87:2621–2627
Dudus L, Anand V, Acland GM, Chen SJ, Wilson JM, Fisher KJ, Maguire AM, Bennett J (1999) Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vis Res 39:2545–2553
Fayad JN, Linthicum FH Jr (2006) Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope 116:1310–1320
Fox J, Weisberg S (2011) An {R} companion to applied regression, Second edn. Sage, Thousand Oaks CA
Guy J, Qi X, Muzyczka N, Hauswirth WW (1999) Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol 117:929–937
Heilbronn R, Weger S (2010) Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol 197:143–170
Hinojosa R, Marion M (1983) Histopathology of profound sensorineural deafness. Ann N Y Acad Sci 405:459–484
Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE (2010) Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol 11:245–265
Kim JR, Abbas PJ, Brown CJ, Etler CP, O'Brien S, Kim LS (2010) The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol 31:1041–1048
Kita Y, Nogimura H, Ida M, Ozawa Y, Kageyama Y, Ohi S, Ito Y, Matsushita K, Takahashi T, Suzuki K, Kazui T, Hayashi S, Enosawa S, Li XK, Suzuki S (2004) Time course of gene expression after the injection of adenoviral vectors containing CTLA4IG gene. Transplant Proc 36:2443–2445
Lalwani AK, Walsh BJ, Carvalho GJ, Muzyczka N, Mhatre AN (1998) Expression of adeno-associated virus integrated transgene within the mammalian vestibular organs. Am J Otol 19:390–395
Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O (2011) Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 519:1526–1545
Leake PA, Hradek GT, Snyder RL (1999) Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol 412:543–562
Miller JM, Altschuler RA (1995) Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion cells following deafness. Ann Otol Rhinol Laryngol Suppl 166:57–60
Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA (1997) Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Internat J Devel Neurosci 15:631–643
Nadol JB Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT Jr, Shallop JK (2001) Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 110:883–891
Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL (2011) Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am 130:3954–3968
Pfingst BE, Hughes AP, Colesa DJ, Watts MM, Strahl SB, Raphael Y (2015) Insertion trauma and recovery of function after cochlear implantation: evidence from objective functional measures. Hear Res 330:98–105
Core Team R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org
Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W (2014) Auditory nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol 15:187–202
Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y (2007) Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res 228:180–187
Searchfield GD, Munoz DJ, Thorne PR (2004) Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear Res 192:23–35
Shepherd RK, Coco A, Epp SB, Crook JM (2005) Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 486:145–158
Shepherd RK, Coco A, Epp SB (2008) Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res 242:100–109
Shibata SB, Budenz CL, Bowling SA, Pfingst BE, Raphael Y (2011) Nerve maintenance and regeneration in the damaged cochlea. Hear Res 281:56–64
Su GL, Colesa DJ, Pfingst BE (2008) Effects of deafening and cochlear implantation procedures on post-implantation psychophysical electrical detection thresholds. Hear Res 241:64–72
Warnock JN, Daigre C, Al-Rubeai M (2011) Introduction to viral vectors. Method Mol Biol 737:1–25
Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT (2010) Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther 18:1111–1122
Zhou N, Kraft CT, Colesa DJ, Pfingst BE (2015) Integration of pulse trains in humans and guinea pigs with cochlear implants. J Assoc Res Otolaryngol 16:523–534
Zhou N, Pfingst BE (2014) Relationship between multipulse integration and speech recognition with cochlear implants. J Acoust Soc Am 136:1257–1268
Zhou N, Xu L, Pfingst BE (2012) Characteristics of detection thresholds and maximum comfortable loudness levels as a function of pulse rate in human cochlear implant users. Hear Res 284:25–32
Acknowledgements
This work was supported by NIH grants R01 DC007634, R01 DC010412, R56 DC010786, R01 DC015809, and P30 DC 005188, the University of Michigan Center for Organogenesis, and a contract from MED-EL. We express appreciation to Cameron Budenz and James Wiler for assistance with AAV inoculations, Lisa Kabara and Melissa Watts for assistance with the electrophysiology, and Lisa Beyer for histological preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Disclosure Statement
One of the authors, Stefan B. Strahl, is an employee of MED-EL GmbH, which was one of the sponsors of this research. None of the other authors have any financial relationship with any of the sponsors.
Electronic supplementary material
Fig. S1
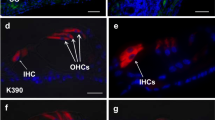
In a set of animals not formally included in this study, we tested the pattern of transduction of an AAV2 vector with a reporter gene (AAV.GFP) and determined that 20 days after the vector was injected into the perilymph of mature deafened guinea pigs, GFP-labeled cells (arrows) were sparsely distributed in the mesothelium lining the scala tympani in both the apical and basal areas of the cochlear partition. GFP was seen in the basal turn (a and b) and as far apically as the third turn (c). In every whole-mount inspected, a small number of cells was transduced, indirectly suggesting that transduction with an AAV2 carrying the Ntf3 gene leads to low levels of NT-3 secreted into cochlear fluids. In a control ear that was not injected with the virus, GFP positive cells were absent (d). These whole-mount preparations include the bone surrounding the osseous spiral lamina which introduces a low level of auto-fluorescence seen in all images. All panels are the same scale (bar = 20 μm). (GIF 101 kb).
Rights and permissions
About this article
Cite this article
Pfingst, B.E., Colesa, D.J., Swiderski, D.L. et al. Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. JARO 18, 731–750 (2017). https://doi.org/10.1007/s10162-017-0633-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0633-9