Abstract
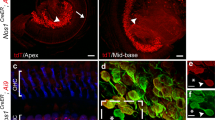
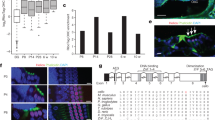
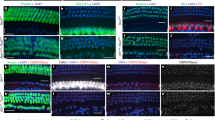
Acoustic information propagates from the ear to the brain via spiral ganglion neurons that innervate hair cells in the cochlea. These afferents include unmyelinated type II fibers that constitute 5 % of the total, the majority being myelinated type I neurons. Lack of specific genetic markers of type II afferents in the cochlea has been a roadblock in studying their functional role. Unexpectedly, type II afferents were visualized by reporter proteins induced by tyrosine hydroxylase (TH)-driven Cre recombinase. The present study was designed to determine whether TH-driven Cre recombinase (TH-2A-CreER) provides a selective and reliable tool for identification and genetic manipulation of type II rather than type I cochlear afferents. The “TH-2A-CreER neurons” radiated from the spiral lamina, crossed the tunnel of Corti, turned towards the base of the cochlea, and traveled beneath the rows of outer hair cells. Neither the processes nor the somata of TH-2A-CreER neurons were labeled by antibodies that specifically labeled type I afferents and medial efferents. TH-2A-CreER-positive processes partially co-labeled with antibodies to peripherin, a known marker of type II afferents. Individual TH-2A-CreER neurons gave off short branches contacting 7–25 outer hair cells (OHCs). Only a fraction of TH-2A-CreER boutons were associated with CtBP2-immunopositive ribbons. These results show that TH-2A-CreER provides a selective marker for type II versus type I afferents and can be used to describe the morphology and arborization pattern of type II cochlear afferents in the mouse cochlea.







Similar content being viewed by others
References
Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL (2002) Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol 447:331–350
Berglund AM, Brown MC (1994) Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res 75:121–130
Berglund AM, Ryugo DK (1987) Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol 255:560–570
Brown MC (1987) Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol 260:605–618
Brown MC (1994) Antidromic responses of single units from the spiral ganglion. J Neurophysiol 71:1835–1847
Brown MC, Berglund AM, Kiang NY, Ryugo DK (1988) Central trajectories of type II spiral ganglion neurons. J Comp Neurol 278:581–590
Brown MC, Ledwith JV 3rd (1990) Projections of thin (type-II) and thick (type-I) auditory-nerve fibers into the cochlear nucleus of the mouse. Hear Res 49:105–118
Darrow KN, Simons EJ, Dodds L, Liberman MC (2006) Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol 498:403–414
Druckenbrod NR, Goodrich LV (2015) Sequential retraction segregates SGN processes during target selection in the cochlea. J Neurosci 35:16221–16235
Dunn RA, Morest DK (1975) Receptor synapses without synaptic ribbons in the cochlea of the cat. Proc Natl Acad Sci U S A 72:3599–3603
Escurat M, Djabali K, Gumpel M, Gros F, Portier MM (1990) Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (NF-L), during the development of the rat. J Neurosci 10:764–784
Eybalin M, Charachon G, Renard N (1993) Dopaminergic lateral efferent innervation of the guinea-pig cochlea: immunoelectron microscopy of catecholamine-synthesizing enzymes and effect of 6-hydroxydopamine. Neuroscience 54:133–142
Fiala JC (2005) Reconstruct: a free editor for serial section microscopy. J Microsc 218:52–61
Flores-Otero J, Davis RL (2011) Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons. J Comp Neurol 519:1455–1475
Flores EN, Duggan A, Madathany T, Hogan AK, Marquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, Garcia-Anoveros J (2015) A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 25:606–612
Francis HW, Nadol JB Jr (1993) Patterns of innervation of outer hair cells in a chimpanzee: I. Afferent and reciprocal synapses. Hear Res 64:184–190
Froud KE, Wong AC, Cederholm JM, Klugmann M, Sandow SL, Julien JP, Ryan AF, Housley GD (2015) Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun 6:7115
Fuchs PA, Glowatzki E (2015) Synaptic studies inform the functional diversity of cochlear afferents. Hear Res 330:18–25
Fuchs PA, Lehar M, Hiel H (2014) Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol 522:717–729
Ginzberg RD, Morest DK (1983) A study of cochlear innervation in the young cat with the Golgi method. Hear Res 10:227–246
Gorham JD, Baker H, Kegler D, Ziff EB (1990) The expression of the neuronal intermediate filament protein peripherin in the rat embryo. Brain Res Dev Brain Res 57:235–248
Hafidi A (1998) Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res 805:181–190
Hafidi A, Despres G, Romand R (1993) Ontogenesis of type II spiral ganglion neurons during development: peripherin immunohistochemistry. Int J Dev Neurosci 11:507–512
Holloway BB, Stornetta RL, Bochorishvili G, Erisir A, Viar KE, Guyenet PG (2013) Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J Neurosci 33:18792–18805
Huang LC, Thorne PR, Housley GD, Montgomery JM (2007a) Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development
Huang LC, Thorne PR, Housley GD, Montgomery JM (2007b) Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 134:2925–2933
Jagger DJ, Housley GD (2003) Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J Physiol 552:525–533
Liberman MC, Dodds LW, Pierce S (1990) Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol 301:443–460
Liu C, Glowatzki E, Fuchs PA (2015) Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci U S A 112:14723–14727
Martinez-Monedero, R. 2016. GluA2-containing AMPA receptors distinguish ribbon-associated from ribbonless afferent contacts on rat cochlear hair cells. 3.
Martinez-Monedero R, Liu C, Weisz C, Vyas P, Fuchs PA, Glowatzki E (2016) GluA2-containing AMPA receptors distinguish ribbon-associated from ribbonless afferent contacts on rat cochlear hair cells. eNeuro 3
McLean WJ, Smith KA, Glowatzki E, Pyott SJ (2009) Distribution of the Na, K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J Assoc Res Otolaryngol 10:37–49
Morgan YV, Ryugo DK, Brown MC (1994) Central trajectories of type II (thin) fibers of the auditory nerve in cats. Hear Res 79:74–82
Mou K, Adamson CL, Davis RL (1998) Time-dependence and cell-type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol 402:129–139
Nadol JB Jr (1983) Serial section reconstruction of the neural poles of hair cells in the human organ of Corti. II. outer hair cells. Laryngoscope 93:780–791
Perkins RE, Morest DK (1975) A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarkski Optics. J Comp Neurol 163:129–158
Reid MA, Flores-Otero J, Davis RL (2004) Firing patterns of type II spiral ganglion neurons in vitro. J Neurosci 24:733–742
Robertson D (1984) Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res 15:113–121
Robertson D, Sellick PM, Patuzzi R (1999) The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res 136:151–158
Rusznak Z, Szucs G (2009) Spiral ganglion neurones: an overview of morphology, firing behaviour, ionic channels and function. Pflugers Arch 457:1303–1325
Ryugo DK, Dodds LW, Benson TE, Kiang NY (1991) Unmyelinated axons of the auditory nerve in cats. J Comp Neurol 308:209–223
Simmons DD, Liberman MC (1988) Afferent innervation of outer hair cells in adult cats: II. Electron microscopic analysis of fibers labeled with horseradish peroxidase. J Comp Neurol 270:145–154
Sobkowicz HM, Rose JE, Scott GL, Levenick CV (1986) Distribution of synaptic ribbons in the developing organ of Corti. J Neurocytol 15:693–714
Sundaresan S, Kong JH, Fang Q, Salles FT, Wangsawihardja F, Ricci AJ, Mustapha M (2016) Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses. Eur J Neurosci 43:148–161
Trigueiros-Cunha N, Renard N, Humbert G, Tavares MA, Eybalin M (2003) Catecholamine-independent transient expression of tyrosine hydroxylase in primary auditory neurons is coincident with the onset of hearing in the rat cochlea. Eur J Neurosci 18:2653–2662
Ugrumov MV (2009) Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat 38:241–256
Wang Q, Green SH (2011) Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci 31:7938–7949
Weisz C, Glowatzki E, Fuchs P (2009) The postsynaptic function of type II cochlear afferents. Nature 461:1126–1129
Weisz CJ, Glowatzki E, Fuchs PA (2014) Excitability of type II cochlear afferents. J Neurosci 34:2365–2373
Weisz CJ, Lehar M, Hiel H, Glowatzki E, Fuchs PA (2012) Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. J Neurosci 32:9528–9536
Xenias HS, Ibanez-Sandoval O, Koos T, Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci 35:6584–6599
Xing Y, Samuvel DJ, Stevens SM, Dubno JR, Schulte BA, Lang H (2012) Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS One 7, e34500
Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM (2002) Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett 523:68–72
Acknowledgments
Supported by NIDCD R01DC006476 and R01DC012957 to EG, NIDCD R01DC011741 to PAF, NIDCD P30 DC005211 to the Center for Hearing and Balance, NS050274 to the Department of Neuroscience Multiphoton Imaging Core, and by support from by the John Mitchell, Jr. Trust and the David M. Rubenstein Fund for Hearing Research. We thank H. Hiel and M. Lehar for the help with electron microscopy. AZ was supported by the Howard Hughes Medical Institute.
Author Contributions
PV, JSW, and EG designed research; PV and JSW performed research and analyzed data; AZ designed and produced the Th 2a-CreER mouse; and PV, JSW, PF, and EG discussed results and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Pankhuri Vyas and Jingjing Sherry Wu contributed equally to this work.
Paul Fuchs and Elisabeth Glowatzki contributed equally to this work.
Rights and permissions
About this article
Cite this article
Vyas, P., Wu, J.S., Zimmerman, A. et al. Tyrosine Hydroxylase Expression in Type II Cochlear Afferents in Mice. JARO 18, 139–151 (2017). https://doi.org/10.1007/s10162-016-0591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0591-7