Abstract
Cochlear function changes throughout the human lifespan. Distortion product otoacoustic emissions (DPOAEs) were recorded in 156 ears to examine these changes and speculate as to their mechanistic underpinnings. DPOAEs were analyzed within the context of current OAE generation theory, which recognizes distinct emission mechanisms. Seven age groups including premature newborns through senescent adults were tested with a swept-tone DPOAE protocol to examine magnitude and phase features of both the mixed DPOAE and individual distortion and reflection components. Results indicate (1) 6–8-month-old infants have the most robust DPOAE and component levels for frequencies >1.5 kHz; (2) older adults show a substantial reduction in DPOAE and distortion-component levels combined with a smaller drop in reflection-component levels; (3) all age groups manifest a violation of distortion phase invariance at frequencies below 1.5 kHz consistent with a secular break in cochlear scaling; the apical phase delay is markedly longer in newborns; and (4) phase slope of reflection emissions is most shallow in the older adults. Combined findings suggest that basilar membrane motion in the apical half of the cochlea is immature at birth and that the cochlea of senescent adults shows reduced nonlinearity and relatively shallow reflection-component phase slope, which can be interpreted to suggest degraded tuning.












Similar content being viewed by others
References
Abdala C (1996) Distortion product otoacoustic emission (2f 1–f 2) amplitude as a function of f 2/f 1 frequency ratio and primary tone level separation in human adults and neonates. J Acoust Soc Am 100:3726–3740
Abdala C (1998) A developmental study of distortion product otoacoustic emission (2f1–f2) suppression in humans. Hear Res 121:125–138
Abdala C (2000) Distortion product otoacoustic emission (2f1–f2) amplitude growth in human adults and neonates. J Acoust Soc Am 107:446–456
Abdala C, Dhar S (2010) DPOAE phase and component analysis in human newborns. J Acoust Soc Am 127:316–325
Abdala C, Keefe D (2006) Effects of middle-ear immaturity on DPOAE suppression tuning in infant ears. J Acoust Soc Am 120:3832–3842
Abdala C, Keefe D, Oba S (2007) Distortion product otoacoustic emission suppression tuning and acoustic admittance in human infants: birth through six months. J Acoust Soc Am 121:3617–3627
Abdala C, Mishra SK, Williams TL (2009) Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex. J Acoust Soc Am 125:1584–1594
Abdala C, Dhar S, Kalluri R (2011a) Level dependence of distortion product otoacoustic emission phase is attributed to component mixing. J Acoust Soc Am 129:3123–3133
Abdala C, Dhar S, Mishra S (2011b) The breaking of cochlear scaling symmetry in human newborns and adults. J Acoust Soc Am 129:1584–1594
Bentsen T, Harte M, Dau T (2011) Human cochlear tuning estimates from stimulus-frequency otoacoustic emissions. J Acoust Soc Am 129:3797–3807
Bonfils P, Uziel A, Narcy P (1989) The properties of spontaneous and evoked otoacoustic emissions in neonates and children: a preliminary report. Arch Otorhinolaryngol 246:249–251
Brown D, Kimberley B, Eggermont J (1994) Cochlear traveling-wave delays estimated by distortion-product emissions in normal hearing adults and term-born neonates. J Otolaryngol 23:234–238
Brown AM, Sheppard SL, Russell PT (1995) Differences between neonatea and adult cochlear mechanical responses. Auditory Neurosci 1:169–181
Burns EM, Arehart KH, Campbell SL (1992) Prevalence of spontaneous otoacoustic emissions in neonates. J Acoust Soc Am 91:1571–1575
Cleveland WS (1993) Visualizing data. Hobart, Summit, pp 88–101
Collet L, Gartner M, Moulin A, Kauffmann I, Disant F, Morgon A (1989) Evoked otoacoustic emissions and sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 115:1060–1062
Cooper N, Rhode W (1995) Nonlinear mechanisms at the apex of the guinea pig cochlea. Hear Res 82:225–243
Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM (1998) Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The epidemiology of hearing loss study. Am J Epidemiol 148:879–886
Deeter R, Abel R, Calandruccio L, Dhar S (2009) Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J Acoust Soc Am 126:2413–2424
Dhar S, Abdala C (2007) A comparative study of distortion-product-emission fine structure in human newborns and adults with normal hearing. J Acoust Soc Am 122:2191–2202
Dhar S, Long GR, Talmadge CL, Tubis A (2005) The effect of stimulus-frequency ratio on distortion product otoacoustic emission components. J Acoust Soc Am 117:3766–3776
Dhar S, Rogers A, Abdala C (2011) Breaking away: violation of distortion emission phase-frequency invariance at low frequencies. J Acoust Soc Am 129:3115–3122
Dong W, Olson ES (2010) Local cochlear damage reduces local nonlinearity and decreases generator-type cochlear emissions while increasing reflector-type emissions. J Acoust Soc Am 127:1422–1431
Dorn PA, Piskorski P, Keefe DH, Neely ST, Gorga MP (1998) On the existence of an age/threshold/frequency interaction in distortion product otoacoustic emissions. J Acoust Soc Am 104:964–971
Engdahl B, Kemp DT (1996) The effect of noise exposure on the details of distortion product otoacoustic emissions in humans. J Acoust Soc Am 99:1573–1587
Gordon-Salant S (2005) Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev 42(4 Suppl 2):9–24
Greenwood DD (1990) A cochlear frequency-position function for several species -29 years later. J Acoust Soc Am 87:2592–2605
Grose JH, Hall JW III, Buss E (2006) Temporal processing deficits in the pre-senescent auditory system. J Acoust Soc Am 119:2305–2315
Harris KC, Ecker MA, Ahlstrom JB, Dubno JR (2010) Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear Res 264:21–29
He NA, Schmiedt RA (1996) Effects of aging on fine structure of the 2f1-f2 acoustic distortion product. J Acoust Soc Am 99:1002–1015
Henin S, Thompson S, Abdelrazeq S, Long GR (2011) Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am 129:2068–2079
Hoth S, Gucmundsdottir K, Plinkert P (2010) Age dependence of otoacoustic emissions: the loss of amplitude is primarily caused by age-related hearing loss and not aging alone. Eur Arch Otorhinolaryngol 267:679–690
Kalluri R, Shera CA (2001) Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am 109:622–637
Kalluri R, Shera CA (2007) Near equivalence of human click-evoked and stimulus frequency otoacoustic emissions. J Acoust Soc Am 121:2097–2110
Kalluri R, Abdala C, Mishra S, Gharibian L (2011) Stimulus-frequency otoacoustic emissions in human newborns. In Poster Presented to the Thirty-Fourth Annual Midwinter Research Meeting of the Association for Research in Otolaryngology, Baltimore, No. 369, pp. 121–122
Keefe DH, Abdala C (2007) Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am 121:978–993
Keefe DH, Abdala C (2011) Distortion-product otoacoustic-emission suppression tuning in human infants and adults using absorbed sound power. J Acoust Soc Am 129:EL108–EL113
Kimberley BP, Brown DK, Eggermont JJ (1993) Measuring human cochlear traveling wave delay using distortion product emission phase responses. J Acoust Soc Am 94:1343–1350
Knight RD, Kemp DT (2000) Indications of different distortion product otoacoustic emission mechanisms from a detailed f1, f2 area study. J Acoust Soc Am 107:457–473
Knight RD, Kemp DT (2001) Wave and place fixed DPOAE maps of the human ear. J Acoust Soc Am 109:1513–1525
Kuroda T (2007) Clinical investigation on spontaneous otoacoustic emission (SOAE) in 447 ears. Auris Nasus Larynx 34:29–38
Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA (2010) Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis. J Assoc Res Otolaryngol 11:419–434
Lasky RE, Perlman J, Hecox K (1992) Distortion-product otoacoustic emissions in human newborns and adults. Ear Hear 13:430–441
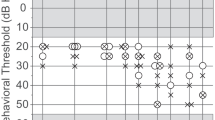
Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee J, Zecker S, Siegel JH (2012) Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear, Mar 20 [Epub ahead of print]
Long GR, Talmadge CL, Lee J (2008) Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am 124:1613–1626
Lonsbury-Martin BL, Cutler WM, Martin GK (1991) Evidence for the influence of aging on distortion-product otoacoustic emissions in humans. J Acoust Soc Am 89:1749–1759
Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN (2011) Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol 12:711–717
Martin GK, Stagner BB, Fahey PF, Lonsbury-Martin BL (2009) Steep and shallow phase gradient distortion product otoacoustic emissions arising basal to the primary tones. J Acoust Soc Am 125:EL 85–EL 92
Mauermann M, Kollmeier B (2004) Distortion product otoacoustic emission (DPOAE) input/output functions and the influence of the second DPOAE source. J Acoust Soc Am 116:2199–2212
Mills DM, Schmiedt RA (2004) Metabolic presbycusis: differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. J Assoc Res Otolaryngol 5:1–10
Morlet T, Lapillonne A, Ferber C, Duclaux R, Sann L, Putet G, Salle B, Collet L (1995) Spontaneous otoacoustic emissions in preterm neonates: prevalence and gender effects. Hear Res 90:44–54
Norton SJ, Widen J (1990) Evoked otoacoustic emissions in normal-hearing infants and children: emerging data and issues. Ear Hear 11:121–127
Oeken J, Lenk A, Bootz E (2000) Influence of age and presbycusis on DPOAE. Acta Otolaryngol 120:396–403
Ohlemiller KK (2004) Age-related hearing loss: the status of Schuknecht's typology. Curr Opin Otolaryngol Head Neck Surg 12:439–443
Prieve BA (1992) Otoacoustic emissions in infants and children: basic characteristics and clinical application. Semin Hear 13:37–52
Pujol R, Lavigne-Rebillard M, Lenoir M (1998) In: Rube E, Popper A, Fay R (eds) Development of sensory and neural structures in the mammalian cochlea, in development of the auditory system. Springer, New York, pp 146–192
Puria S (2003) Measurements of human middle ear forward and reverse acoustics: implications for otoacoustic emissions. J Acoust Soc Am 113:2773–2789
Rao A, Long GR (2011) Effects of aspirin on distortion product fine structure: interpreted by the two-source model for distortion product otoacoustic emissions generation. J Acoust Soc Am 129:792–800
Rhode WS (1971) Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. J Acoust Soc Am 49(Suppl 2):1218
Ruggero MA, Rich NC (1991) Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci 11:1057–1067
Ruggero MA, Rich NC, Recio A (1996) The effect of intense acoustic stimulation on basilar-membrane vibration. Aud Neurosci 2:329–345
Schairer KS, Ellison JC, Fitzpatrick D, Keefe DH (2006) Use of stimulus frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears. J Acoust Soc Am 120:901–914
Schmiedt R, Lang H, Okamura H, Schulte B (2002) Effects of furosemide applied chronically to the round window: a model of metabolic presbycusis. J Neurosci 22:9643–9650
Schuknecht HF (1955) Presbycusis. Laryngoscope 65:402–419
Shaffer LA, Dhar S (2006) DPOAE component estimates and their relationship to hearing thresholds. J Am Acad Audiol 17:279–292
Shera CA, Guinan JJ Jr (1999) Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105:782–798
Shera CA, Guinan JJ (2003) Stimulus frequency-emissions group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am 113:2762–2772
Shera CA, Talmadge CL, Tubis A (2000) Interrelations among distortion-product phase-gradient delays: their connection to scaling symmetry and its breaking. J Acoust Soc Am 108:2933–2948
Shera CA, Guinan JJ, Oxenham AJ (2010) Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol 11:343–365
Smurzynski J, Jung MD, Lafreniere D, Kim DO, Kamath MV, Rowe JC, Holman MC, Leonard G (1993) Distortion-product and click-evoked otoacoustic emissions of preterm and full-term infants. Ear Hear 14:258–274
Stover L, Norton SJ (1993) The effects of aging on otoacoustic emissions. J Acoust Soc Am 94:2670–2681
Strickland EA, Burns EM, Tubis A (1985) Incidence of spontaneous otoacoustic emissions in children and infants. J Acoust Soc Am 78:931–935
Talmadge CL, Tubis A, Long GR, Piskorski P (1998) Modeling otoacoustic emission and hearing threshold fine structures. J Acoust Soc Am 104:1517–1543
Talmadge CL, Long GR, Tubis A, Dhar S (1999) Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J Acoust Soc Am 105:275–292
Uchida Y, Ando F, Shimokata H, Sugiura S, Ueda H, Nakashima T (2008) The effects of aging on distortion product otoacoustic emissions in adults with normal hearing. Ear Hear 29:176–184
Zweig G (1976) Basilar membrane motion. Cold Spring Harb Symp Quant Biol 40:619–633
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98:2018–2047
Acknowledgments
This work was supported by the National Institutes of Health R01DC003552 and the House Research Institute. The authors would like to thank Srikanta Mishra, Shannon Luckovich (HRI), Angela Garinis (UW), Abigail Rogers, and Sumaya Sidique (NU) for data collection and Silvia Batezati, Laurel Fisher, and Ping Luo for assistance with data management. We appreciate helpful discussions with Bob Shannon and Chris Shera and thank Dr. Shera for his loess modeling script.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdala, C., Dhar, S. Maturation and Aging of the Human Cochlea: A View through the DPOAE Looking Glass. JARO 13, 403–421 (2012). https://doi.org/10.1007/s10162-012-0319-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-012-0319-2