Abstract
Otoferlin is involved in neurotransmitter release at the synapse between inner hair cells (IHCs) and auditory nerve fibres, and mutations in the OTOF gene result in severe to profound hearing loss. Abnormal sound-evoked cochlear potentials were recorded with transtympanic electrocochleography from four children with otoferlin (OTOF) mutations to evaluate physiological effects in humans of abnormal neurotransmitter release from IHCs. The subjects were profoundly deaf with absent auditory brainstem responses and preserved otoacoustic emissions consistent with auditory neuropathy. Two children were compound heterozygotes for mutations c.2732_2735dupAGCT and p.Ala964Glu; one subject was homozygous for mutation p.Phe1795Cys, and one was compound heterozygote for two novel mutations c.1609delG in exon 16 and c.1966delC in exon 18. Cochlear potentials evoked by clicks from 60 to 120 dB peak equivalent sound pressure level were compared to recordings obtained from 16 normally hearing children. Cochlear microphonic (CM) was recorded with normal amplitudes from all but one ear. After cancelling CM, cochlear potentials were of negative polarity with reduced amplitude and prolonged duration compared to controls. These cochlear potentials were recorded as low as 50–90 dB below behavioural thresholds in contrast to the close correlation in controls between cochlear potentials and behavioural threshold. Summating potential was identified in five out of eight ears with normal latency whilst auditory nerve compound action potentials were either absent or of low amplitude. Stimulation at high rates reduced amplitude and duration of the prolonged potentials, consistent with neural generation. This study suggests that mechano-electrical transduction and cochlear amplification are normal in patients with OTOF mutations. The low-amplitude prolonged negative potentials are consistent with decreased neurotransmitter release resulting in abnormal dendritic activation and impairment of auditory nerve firing.
Similar content being viewed by others
Introduction
Current knowledge indicates that over 60% of cases of hearing impairment are related to genetic causes (Petit et al. 2001). It has been estimated that mutations in the OTOF gene are responsible for at least 3% of cases of pre-lingual non-syndromic hearing impairment in the Spanish population (Rodríguez-Ballesteros et al. 2008). The OTOF gene encodes otoferlin, a transmembrane protein belonging to the ferlin protein family, which contains several repeating C2 domains involved in calcium binding (Rizo and Südhof 1998; Jiménez and Bashir 2007). Studies performed in mice indicate that otoferlin plays a crucial role in vesicle release at the synapse between inner hair cells (IHCs) and auditory nerve fibres by interacting with syntaxin1 and SNAP25 (Roux et al. 2006).
To date, 42 pathogenic mutations of the OTOF gene have been identified (Rodríguez-Ballesteros et al. 2008 for review), all resulting in a very homogeneous phenotype of pre-lingual profound hearing loss. Accordingly, recordings of auditory evoked potentials have shown the absence or the marked threshold elevation of auditory brainstem responses (ABRs). Moreover, over 50% of subjects with OTOF mutations also show otoacoustic emissions (OAEs; Rodriguez-Ballesteros et al. 2003; 2008). The association of absence of ABRs and detection of OAEs is typical of a clinical disorder known as auditory neuropathy (AN) which is characterised by disruption of auditory nerve activity with preservation of outer hair cell (OHC) function (Starr et al. 1996). AN has a wide range of aetiologies (genetic, infectious, toxic–metabolic, immunological, etc.) and occurs in all age groups (Starr et al. 2000; Rapin and Gravel 2003). It has been hypothesised that the disorder results from impaired function of auditory nerve fibres due to demyelination and axonal loss (post-synaptic; Starr et al. 2003; Rapin and Gravel 2003; Santarelli et al. 2008) or lesions involving IHCs and/or the synapses with auditory nerve fibres (pre-synaptic; Starr et al. 2004; McMahon et al. 2008; Santarelli et al. 2008). Studies performed in otoferlin-deficient mice (Roux et al. 2006) have shown the absence or marked threshold elevation of ABRs together with the preservation of OAEs. These findings would appear to be consistent with a defect in synaptic vesicle release at the pre-synaptic membrane of IHCs (Roux et al. 2006).
Whereas detection of OAEs may indicate preserved OHC function, the physiological consequences of a lesion involving the afferent component of the auditory periphery cannot be evaluated effectively by far-field recording techniques such as ABR due to the low signal-to-noise ratio (Starr 2001; Santarelli et al. 2002, 2006). Both receptor (cochlear microphonic, CM; summating potential, SP) and auditory nerve activity (compound action potential, CAP) can be evaluated using a near-field recording technique such as transtympanic electrocochleography (ECochG; Eggermont and Odenthal 1974a). Recently, ECochG potentials have been recorded from children and young adults affected by AN (McMahon et al. 2008; Santarelli et al. 2008). Cochlear microphonics, which are believed to arise almost exclusively from OHCs in the basal portion of the cochlea (Dallos and Wang 1974; Withnell 2001), were normal. Moreover, in our study (Santarelli et al. 2008), the cochlear potentials obtained after CM cancellation from one group of subjects with AN consisted only of the SP not followed by a CAP. Since SP is acknowledged to be generated primarily by IHCs in the basal portion of the cochlea (Zheng et al. 1997; Durrant et al. 1998), this picture was suggested as being consistent with pre-synaptic IHC disorder. In other patients, the SP was normal and was followed by a delayed CAP or sustained low-amplitude negative activity consistent with a dendritic origin (Santarelli et al. 2008).
In this study, we evaluate receptor and neural cochlear potentials obtained by transtympanic ECochG from four children with biallelic mutations in the OTOF gene so as to define physiological cochlear functions which accompany this pre-synaptic disorder involving the IHCs.
Methods
Subjects
Four children (two males and two females) ranging in age from 12 to 20 months at the time of ECochG recording were included in this study. All subjects had passed neonatal hearing screening with OAE recording. They were referred for assessment because of parental concern regarding the children’s hearing. Subjects 1 and 3 were brother and sister. The younger subject, the sister, was referred at an earlier age (1 month) than her brother (12 months). Growth and motor development were normal in all four children. Both CT and MRI head and ear (including internal acoustic canal) scans (gadolinium infusion) were normal. All patients had undergone cochlear implantation by the age of 2.
The results reported in this study are from a retrospective analysis. Children were subjected to ECochG recording as part of our standard assessment protocol for cochlear implantation. Parents signed a written informed consent both for the anaesthesia and the ECochG recording. ECochG protocol was assessed by the regional body for quality control of clinical and therapeutic procedures (CCHSA, Veneto Region 2007-2010), and medical–electrical equipment used for recording evoked potentials satisfied the safety requirements of the International Standard IEC 60601-1.
Blood samples for genetic analysis were collected after completion of ECochG procedures and before general anaesthesia was discontinued. A second informed consent form was signed by parents allowing genetic analysis to be performed and the results to be used for research purposes. Ten children with the clinical picture of AN without risk factors for hearing loss apart from familiarity underwent OTOF analysis. Of these, only four proved to have OTOF gene mutations. Three have previously been included in the study by Rodríguez-Ballesteros et al. (2008).
Cochlear potentials recorded in patients with OTOF mutations were compared to the ECochG data previously collected from 16 children (mean 3.7 years, range 3.5–6.5 years) tested for presumed cochlear deafness but who proved to have normal cochlear function with normal thresholds when evoking neural and receptor potentials (Santarelli et al. 2008). Normal hearing thresholds, as evaluated by visual reinforcement audiometry, were obtained from four children included in the control group 2–3 years after the ECochG recording session (Table 1). Children with OTOF mutations were younger than controls. We do not, however, consider the age difference to be a major limitation. Although no data are available to suggest that cochlear receptor and neural potentials change with age in humans, the latency of ABR wave I evaluated by narrow band noise techniques is comparable to adult values by the age of 1–2 years (Eggermont et al. 1991). Furthermore, no significant differences in distortion product otoacoustic emission (DPOAE) amplitude have been observed amongst children aged from 1 to 5 years (Prieve et al. 1997).
Genetic study
DNA was extracted from peripheral blood samples by standard procedures. Primers and conditions for polymerase chain reaction (PCR) amplification and DNA sequencing of each exon of the OTOF gene have been reported (Migliosi et al. 2002; Rodriguez-Ballesteros et al. 2003). Specific tests were developed for the detection of the two novel sequence variants that were found in OTOF during this study. They were based on the mini-sequencing SNaPshot methodology (Applied Biosystems, Foster City, CA, USA). PCR amplifications of exons 16 and 18, containing respectively the c.1609delG and c.1966delC mutations, were carried out as described (Migliosi et al. 2002). Mini-sequencing reactions (primer extension with fluorescently labelled 2′,3′-dideoxynucleotides) were performed by using primer 5′-ACACTGGGCCCAGCCTGG-3′ (for c.1609delG) and primer 5′-TCCCGGCCCCAGCGGCCTCGGCCC-3′ (for c.1966delC), and the reaction products were separated in an ABI 3130 Genetic Analyser, as recommended by the manufacturer (Applied Biosystems, Foster City, CA, USA).
Nomenclature of mutations is based on cDNA sequence (GenBank accession number AF183185.1), the A of the translation initiation codon being considered as +1.
Audiological studies
-
(a)
Pure tone audiometry
Hearing thresholds were evaluated in a sound-attenuated room by visual reinforcement audiometry. Stimuli were warble tones presented in free field at octave frequencies from 250 to 4,000 Hz (Interacoustic AC30 Audiometer connected to a Pioneer A 103 amplifier, JBL TLX130 loudspeakers). The stimulus was calibrated by means of a Brüel and Kjaer 4165 microphone mounted on an 800 B Larson-Davis sound level metre. Degree of hearing impairment was defined by pure tone average (PTA) threshold levels at 0.5, 1, 2 and 4 kHz. Hearing loss was classified as mild (PTA 21–40 dB HL), moderate (PTA 41–70 dB HL), severe (PTA 71–95 dB HL) and profound (PTA > 95 dB HL; European Concerted Action Project on Genetics of Hearing Impairment 1996). When no response was found at 120 dB HL, the threshold was arbitrarily set at 130 dB HL.
Tympanometry and acoustic reflex thresholds were measured with a Grason-Stadler GSI 33 impedance audiometer. Acoustic reflexes were obtained ipsilaterally and contralaterally with respect to stimulus presentation and were considered absent when no response was found at intensities higher than 110 dB HL.
-
(b)
DPOAEs
DPOAEs were obtained using the ILO-92 OAE system (Otodynamics). During recordings of DPOAEs, primary tones f1 and f2 were presented at 70 dB peak equivalent sound pressure level (p.e. SPL), and the f2-to-f1 ratio was kept at 1.21. The frequency was increased in one-fourth octave steps from 708 to 6,299 Hz. Four spectral averages were summed for each stimulus condition.
-
(c)
ABRs
Potentials were recorded from scalp electrodes (vertex to mastoid ipsilateral to the stimulated ear) in response to 2,000 trials of alternating polarity clicks presented monaurally using a TDH-50 transducer earphone at a maximum intensity of 125 dB p.e. SPL (corresponding to 90 dB nHL, referred to the psychoacoustical threshold of normally hearing subjects). The stimulus was calibrated by connecting the transducer to a 6-cm3 coupler (ISO 389). The procedure of comparing the peak-to-peak amplitude of the click to the peak-to-peak amplitude of a 1-kHz tone was used to calibrate the click level (p.e. SPL).
-
(d)
ECochG
Subjects were tested under general anaesthesia (sevoflurane). A sterile stainless steel needle electrode (0.7 mm), insulated except for the tip, was passed through the tympanic membrane and placed on the promontory wall with the aid of an operating microscope. Stimuli consisted of 0.1-ms rarefaction and condensation clicks, separately delivered in free field by means of two high-frequency drivers (Electro-Voice DH1A/2MT 16 Ω) mounted on a single polyurethane horn (Electro-Voice HP420) with a maximum intensity of 120 dB p.e. SPL (corresponding to 90 dB nHL, referred to the psychoacoustical threshold of normally hearing subjects). The stimulus was calibrated in a free field by means of a Brüel and Kjaer 4165 microphone (mounted on an 800 B Larson-Davis sound level metre) placed at 1 m from the base of the polyurethane horn, which corresponded to the distance of the patient’s ear from the horn. The procedure of comparing the peak-to-peak amplitude of the click to the peak-to-peak amplitude of a 2-kHz tone was utilised to calibrate the click level (p.e. SPL).
Condensation and rarefaction clicks were delivered separately for intensity levels from 60 to 120 p.e. SPL. The stimulus paradigm used in three patients (#1–3) and in controls consisted of an initial click, followed 15 ms later by ten clicks with an inter-stimulus interval of 2.9 ms. This sequence was repeated every 191 ms. This stimulus paradigm was used to distinguish between neural and receptor potentials by taking advantage of the different effects of adaptation induced by high stimulation rates (Eggermont and Odenthal 1974b). In the remaining subject (#4), a repetitive click stimulation with an inter-stimulus interval of 91 ms was used.
The potentials were differentially amplified (50,000 times), filtered (5–8,000 Hz) and digitised (25 µs) for averaging (500 trials). The procedure of averaging the responses evoked separately by condensation and rarefaction clicks was applied to cancel the CM and extract the CAP with the superimposed SP. The resulting curve was subtracted from the potential evoked by condensation clicks to obtain the CM (Santarelli et al. 2008). Since CM attenuation was often incomplete when stimulus intensities were higher than 110 dB p.e. SPL and CM spectral energy was at maximum between 1,500 and 3,000 Hz, a low pass digital filter (12 dB per octave, cutoff frequency 2,000 Hz) was used to attenuate the residual CM, where needed.
ECochG response measures
Latency was defined relative to CM onset in milliseconds. Amplitude was computed relative to the period 1 ms before CM onset in microvolt.
Since the SP and CAP could not be identified separately in five ears out of eight, we considered the ECochG response obtained after CM cancellation as a single event (SP–CAP), and defined the SP–CAP onset at the initial negative deflection arising from baseline, SP–CAP peak at maximum negative potential and SP–CAP end at the return to baseline (Santarelli et al. 2008). Analogously, both amplitude and duration changes during adaptation were evaluated on the SP–CAP as a whole and were compared to the corresponding values obtained from controls.
Values contained in the text indicate mean ± standard error.
Results
-
(a)
Genetic study
Mutations in the coding region of the OTOF gene carried by the patients participating in this study are reported in Figure 1. Subjects #1 and #3, brother and sister, are compound heterozygotes for mutations c.2732_2735dupAGCT and p.Ala964Glu. Subject #4 is homozygous for mutation p.Phe1795Cys. We found two novel mutations in the OTOF gene in subject #2. She is compound heterozygote for c.1609delG in exon 16 (p.Val537X) and c.1966delC in exon 18 (p.Arg656GlyfsX10). Both mutations generate premature stop codons and may result in the synthesis of truncated polypeptides or in no otoferlin synthesis at all if the resulting messenger RNAs are degraded by the non-sense-mediated RNA decay pathway. Segregation analysis in this family confirmed that the affected subject carries these two mutations in trans. None of these mutations were found in 50 Italian subjects with normal hearing.
-
(b)
Audiological measures
Mutations in the coding region of the OTOF gene that are carried by the patients participating in this study. Vertical lines indicate the position of each mutation on the reference cDNA (accession number AF183185.1) and on the correlated position in the protein. A horizontal bar represents the cDNA, small vertical bars delimiting the exons, which are numbered. The protein is represented by a rectangle, boxes indicating the predicted domains of the protein. Black boxes, C2 domains. Cross-hatched box, transmembrane domain. Missense mutations are shown in bold.
Hearing loss was profound in all children (Table 1). Mean PTA threshold at 0.5, 1, 2 and 4 kHz was 117.0 ± 8.9 dB HL. ABRs were absent whilst DPOAEs were detected in all ears. All children had absent middle ear muscle acoustic reflexes bilaterally.
-
(c)
Cochlear potentials
ECochG recording of cochlear potentials from subjects with mutations in the OTOF gene showed: (1) normal amplitudes of the receptor CM; (2) absence or abnormally reduced amplitude of the neural CAP; and (3) abnormally prolonged negative potentials decreasing in both amplitude and duration in response to a neural adaptation paradigm.
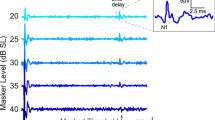
Cochlear potentials (CM, SP–CAP) recorded at 120 dB p.e. SPL in patients with mutations in the OTOF gene are shown in Figure 2 together with the grand average of corresponding control recordings with 95% confidence limits (shadowed area). The left panel contains the CM potentials whilst the cochlear responses resulting from CM cancellation (see “Methods”) are reported on the right. CMs were recorded from all ears with amplitudes within the control range in all but subject #2 who showed an abnormally large response in the right ear (Table 1). The cochlear potentials obtained after CM cancellation in controls show an initial fast SP deflection followed by a CAP returning to baseline by 2.4 ms. Individual recordings obtained from patients with OTOF mutations consisted of negative potentials showing reduced amplitude and prolonged duration compared to controls. SP was identified in five ears, as fast negative deflection arising from baseline at the beginning of the prolonged negative activity with peak latencies (filled triangles) falling in the control range of SP latencies (Table 1). In these ears, SP amplitude was normal (two ears) or reduced (three ears) compared to controls (Table 1). An attenuated CAP was tentatively identified in three ears (right ear in subject #2, both ears in subject #4) as a small negative deflection (filled circles) following the SP potential at peak latencies within the control range of CAP latencies (Table 1). In the remaining five ears, the CAP could not be identified.
CM and SP–CAP potentials recorded from controls and patients with OTOF mutations in response to a high-intensity click stimulus (120 dB p.e. SPL). Mean CM and SP–CAP potentials obtained by averaging all control recordings are reported in the upper part of left and right panel, respectively. The shadowed area refers to 95% confidence limits. Individual CMs and SP–CAPs recorded from the right (R) and left (L) ears in patients with OTOF mutations are reported below. CMs were recorded from all ears with normal amplitudes in all but subject #2 who showed an abnormally large response in the right ear. SP was identified in five ears (SP peak, filled triangles) as the fast negative deflection with peak latencies comparable to mean control SP latencies. A CAP was identified in three ears (CAP peak, filled circles) as the small negative deflection following the initial fast negative SP potential. The vertical dashed lines indicates the mean latency of the SP and CAP peak in controls. The horizontal bar extends from SP–CAP onset to return to baseline. Notice that SP–CAP responses were markedly prolonged compared to controls in all but subject #1. Time “0” refers to CM onset in this and in the subsequent figures.
Due to the lack of separation between SP and CAP in five ears out of eight, we considered the ECochG response resulting from CM cancellation as a single event, designated as SP–CAP (see “Methods”). Overall, the SP–CAP potentials recorded from children with OTOF mutations appeared as negative deflections which were markedly reduced in amplitude and prolonged in duration compared to controls (Table 1). The response duration measured from onset to return to baseline is indicated by the solid horizontal lines along the baseline in Figure 2. It can be seen that SP–CAP potentials were markedly prolonged in all but one ear (right ear, subject #1). Compared to controls, the increase in duration ranged from 3.24 to 9.4 ms as measured at 120 dB p.e. SPL (Table 1).
The effects of signal intensity on SP–CAP potentials are shown for a control and for patient #2 (right ear) in Figure 3. In the control, decreasing stimulus levels resulted in gradual latency increase and amplitude reduction of both SP (filled triangles) and CAP (filled circles) peaks. At 60 dB p.e. SPL, an intensity close to the behavioural threshold for normal-hearing subjects (Eggermont 1976), CAP and SP could not be identified separately and cochlear potentials showed an increased duration compared to 120 dB p.e. SPL (from 2.55 ± 0.13 to 3.30 ± 0.12 ms). The SP–CAP potentials recorded from subject #2 were of low amplitude and prolonged duration compared to the control. The response duration was 12 ms whereas in the control the SP–CAP returned to baseline by 2.2 ms as measured at 120 dB p.e. SPL. At this intensity, the SP recorded from subject #2 was of comparable amplitude to that seen in the control and was followed by a low-amplitude CAP. At stimulus levels from 110 to 90 dB p.e. SPL, the SP–CAP potentials began with a fast negative deflection with amplitude and peak latency comparable to control SP, followed by a low-amplitude sustained negative component. At lower stimulus intensities, only the prolonged negative potential could be identified. This was found as low as 60 dB p.e. SPL, which is 90 dB below the behavioural threshold (PTA 120 dB HL). The identification of the prolonged negative activity below behavioural thresholds occurred in all patients with OTOF mutations in contrast to the close correlation in controls between cochlear potentials and behavioural threshold (compare SP–CAP threshold and PTA in Table 1). Differences between SP–CAP and PTA thresholds ranged from 50 to 90 dB.
SP–CAP potentials recorded from one control and subject #2 (right ear) at decreasing stimulation intensities (120–60 dB p.e. SPL). Filled circles and triangles refer to the CAP and SP peak, respectively. In the control, decreasing stimulus levels resulted in gradual latency increase and amplitude reduction of both SP and CAP peaks. In subject #2, the CAP was identified only at the highest stimulation intensity (120 dB p.e. SPL) whilst the SP was recorded from 120 to 90 dB p.e. SPL. Both CAP and SP were superimposed on a sustained low-amplitude negative potential which was identified as low as 60 dB p.e. SPL.
CM amplitude and SP–CAP peak amplitude, duration and peak latency are reported as a function of signal intensity in Figure 4 for both controls and subjects with OTOF mutations. Mean control values with 95% confidence limits are superimposed at each stimulation intensity on the corresponding individual values obtained from patients with OTOF mutations. CM amplitudes were within the control range for all but subject #2 who showed an abnormally high CM amplitude in the right ear at intensities from 100 to 120 dB p.e. SPL. Compared to controls, SP–CAP amplitude was markedly reduced in all ears whilst SP–CAP duration was prolonged in all but subject #1 who showed response durations within normal limits in the right ear and a slightly prolonged response in the left ear at 120 dB p.e. SPL. SP–CAP latencies varied widely in subjects although values were within or just above the control range in all but one ear (right ear, subject #2).
-
(d)
Adaptation of ECochG potentials
CM amplitude and SP–CAP peak amplitude, duration and peak latency from controls and subjects with OTOF mutations. In each panel, mean values calculated for controls (thick line) with 95% confidence limits (shadowed area) are superimposed on individual values obtained from subjects with OTOF mutations. Compared to controls, patients with OTOF mutations showed prolonged SP–CAP durations and reduced SP–CAP amplitudes whilst CM amplitudes were within the control range for all but subject #2, who showed abnormally large CM in the right ear.
A neural adaptation paradigm produced changes in both amplitude and duration of SP–CAP responses in children with OTOF mutations. We tested adaptation in three (#1, #2, #3) of the subjects, bilaterally in #2 and #3 and unilaterally in #1. In the left panels of Figure 5, the SP–CAP potentials evoked by the last (#11, adapted response) and first click (#1, non- adapted response) of the stimulus sequence at 110 dB p.e. SPL are superimposed for controls and three children with OTOF mutations. The upper panel (A) contains the mean curve obtained by averaging all control recordings. In controls, CAP amplitude was markedly attenuated after adaptation (70% reduction from the first to the 11th click) whereas SP attenuation was much lower, averaging 27% (Fig. 5 A, Table 1). Moreover, response duration as measured from SP onset to return to baseline was unchanged after adaptation (Table 1).
Adaptation of SP–CAP potentials. SP–CAPs evoked by the first (#1) and last (#11) click of the stimulus train (see “Methods” for details) at 110 dB p.e. SPL are displayed for controls and subjects with OTOF mutations (subjects #1, #2 and #3) in the left panel. The mean curves obtained by averaging all control recordings are reported in the upper part (A). In controls, there was a remarkable reduction in CAP amplitude (70%) by the last click of the stimulus train whereas the attenuation of the SP amplitude was much lower (27%). SP–CAP recorded from subject #1 (B, right ear) showed small changes in both amplitude (22%) and duration (1.3 ms) during adaptation. In contrast, SP–CAP potentials recorded from the left ear of both subjects #2 (C) and #3 (D) were remarkably attenuated in amplitude and decreased in duration by the last click of the stimulus sequence. In subject #3, the response attenuation was measured at the second negative peak (arrow), which was within the control CAP latency range. Changes of individual SP–CAP duration (E) and amplitude (F) during adaptation are reported in the right panel. In the upper graph (E), individual SP–CAP durations calculated for patients with OTOF mutations in the baseline condition (click #1) and after adaptation (click #11) are reported together with the corresponding mean values from controls. The response duration decreased to control values during adaptation in subjects #2 and #3 whereas the SP–CAP recorded from subject #1 showed minimal decrease in duration. The lower graph (F) reports the SP–CAP amplitude attenuation in patients with OTOF mutations as a function of click position in the stimulus sequence. Individual values are superimposed on mean SP (closed circles) and SP–CAP attenuation (closed squares) calculated for controls. The profile of SP–CAP attenuation in subject #1 was close to the SP attenuation profile in controls whereas the SP–CAP attenuation calculated for both subjects #2 and #3 closely followed the profile of SP–CAP attenuation in controls.
Due to the lack of separation between SP and CAP and the small CAP amplitude in patients with OTOF mutations, the amplitude changes induced by adaptation were evaluated on the SP–CAP response as a whole rather than on SP and CAP potentials separately. The SP–CAP potential recorded from subject #1 (Fig. 5 B, Table 1) showed only minor changes in peak amplitude (22%) and duration (1.3 ms) in the adapted response. In contrast, the SP–CAP potentials recorded from subjects #2 and #3 (left ear, Fig. 5 C, D) were markedly reduced in amplitude during adaptation and the amount of response attenuation calculated for both ears was within the range of mean SP–CAP attenuations in controls (Table 1). The duration of SP–CAP responses, which was abnormally prolonged in both subjects #2 and #3, dropped to control values during adaptation (Fig. 5 E, Table 1).
SP–CAP attenuations obtained at 110 dB p.e. SPL from subjects with OTOF mutations are reported in Figure 5 F as a function of click position in the stimulus sequence. Values calculated for patients have been superimposed on the profile of mean amplitude attenuation calculated for both SP and SP–CAP in controls. The profile of SP–CAP attenuation obtained from both ears in subjects #2 and #3 clearly followed the profile of mean SP–CAP attenuation in controls. In contrast, SP–CAP attenuation calculated for subject #1 was close to the profile of mean SP attenuation in controls.
Discussion
In this study, we recorded sound-evoked cochlear potentials with transtympanic electrocochleography from four deaf children with OTOF mutations. CM was recorded with normal amplitude from all but one ear. The ECochG response obtained after CM cancellation appeared as a broad negative deflection markedly prolonged in duration and reduced in amplitude compared to controls. SP was identified in five ears at the beginning of these prolonged potentials and was followed by a low-amplitude CAP in three ears. The prolonged negative activity was sensitive to a neural adaptation paradigm as stimulation at high rate produced a striking fall in both amplitude and duration.
CM was of control amplitude in all but one child. These findings together with the detection of DPOAEs point to normal OHC function in accordance with previously reported OAE data from patients with OTOF mutations (Migliosi et al. 2002; Rodríguez-Ballesteros et al. 2008) and otoferlin-deficient mice (Roux et al. 2006). Enhanced CM amplitudes have not been reported so far in patients with OTOF mutations, whilst high-amplitude CM responses have been found in children (Santarelli et al. 2002, 2006) and adults with AN (Starr et al. 2001; Santarelli et al. 2008), and in children with brain damage (Santarelli et al. 2006). In these patients, CM enhancement has been suggested as resulting from decreased activity of the efferent system secondary to abnormal nerve fibre activation or reduced function of IHCs (Starr et al. 2001; Santarelli et al. 2008). The large CM amplitude found in one subject with OTOF mutations might result from reduced nerve fibre activation due to abnormal synaptic release from IHCs.
In patients with OTOF mutations, the cochlear potentials obtained after CM cancellation showed the absence of or substantial reduction in CAP amplitude. Accordingly, ABRs could not be obtained from these children. Also, recordings of auditory evoked potentials in otoferlin-deficient mice (Roux et al. 2006) have shown the absence or the marked threshold elevation of ABRs. Studies performed in these mice (Roux et al. 2006) support the hypothesis that otoferlin plays a crucial role in synaptic vesicle release from IHCs as it acts as a calcium sensor protein enhancing vesicle fusion by interacting with the SNARE proteins syntaxin1 and SNAP25. Particularly, otoferlin may be involved in the fast step of vesicle release whereas the late step of exocytosis is only reduced (Roux et al. 2006). It has been suggested that the absence of ABRs in otoferlin-deficient mice reflects a defect in synaptic vesicle release with little or no post-synaptic membrane activation and consequent impairment of action potential generation (Roux et al. 2006). The absence or significant decrease in the CAP we found in children with OTOF mutations is consistent with this hypothesis, indicating that the effects of the mutations in the OTOF gene in humans may be similar to those found in experimental models lacking otoferlin. However, one unexpected finding in patients with OTOF mutations was the presence of negative low-amplitude prolonged potentials which were recorded as low as 50–90 dB below behavioural thresholds. This response pattern is similar to that described in our previous paper for a subset of patients with AN due to a variety of aetiologies other than mutations in the OTOF gene (Santarelli et al. 2008). Similar findings have been reported by McMahon et al. (2008) for the post-synaptic type of ECochG responses recorded from the round window of children with AN. In both studies, this response has been interpreted as a dendritic potential resulting from local depolarisation of the distal portion of the afferent fibres without spike initiation. Whilst we have no definite explanation for the origin of the prolonged potentials found in subjects with OTOF mutations, the sensitivity of these responses to fast stimulus rates is consistent with their neural generation. In controls, the mean amplitude decrease of neural CAP during adaptation (70%) was much higher than the receptor SP attenuation (27%) whilst there was no change in SP–CAP duration. Due to the lack of separation between SP and CAP in some patients with OTOF mutations, amplitude changes induced by adaptation were evaluated on the SP–CAP as a whole rather than on separate SP and CAP potentials. In controls, the mean attenuation calculated for SP–CAP (56%) was lower than the attenuation measured for the CAP alone (70%) due to the lower SP amplitude decrease during adaptation. The size of the SP–CAP decrease in amplitude during adaptation calculated for patients with OTOF mutations (52–56%) was comparable to the mean SP–CAP attenuation for controls, and this figure was more than twice the mean SP control attenuation. Moreover, SP–CAP duration, which was abnormally prolonged in the non-adapted state, dropped to control values at high stimulation rate. These findings are consistent with a neural origin of the prolonged negative potentials.
The prolonged responses obtained from patients with OTOF mutations seem analogous to the dendritic potentials recorded by Sellick et al. (2003) from the scala tympani of guinea pigs after administration of tetrodotoxin to block neural spiking in terminal dendrites of auditory nerve fibres. It may then be feasible that in patients with OTOF mutations an abnormal and/or reduced post-synaptic activation should generate local dendritic potentials without initiating neural spiking or that local potentials may only occasionally reach the threshold to trigger action potentials recorded as high-threshold CAP. Alternatively, the prolonged potentials may result from abnormally prolonged SPs not followed by a CAP. This hypothesis seems unlikely for several reasons. Firstly, SP potentials recorded in controls show little sensitivity to a neural adaptation paradigm (Santarelli et al. 2008). Moreover, the duration of SP potentials in subjects with normal hearing (Eggermont and Odenthal 1974b) and patients affected by cochlear disorders (Schmidt et al. 1974; Ferraro et al. 1994) is typically restricted to the time of the stimulus when utilising tone burst stimulation (Sellick et al. 2003).
In one child with OTOF mutations (#1), there was no prolongation of SP–CAP response whilst the neural adaptation paradigm failed to produce changes either in amplitude or duration. Similar responses have been recorded from some patients with AN (McMahon et al. 2008; Santarelli et al. 2008), and they have been interpreted as SP potentials. Analogously, local administration of kainic acid disrupting the synapses between IHCs and afferent fibres has resulted in CAP abolition together with SP preservation in chinchillas (Zheng et al. 1997). Compared to other patients included in this study, the electrophysiological pattern observed in subject #1 may represent a more advanced stage of the disorder with complete impairment of vesicle release and little or no activation of afferent fibres.
Whereas receptor CM potentials are easily identifiable in subjects with OTOF mutations, the identification of receptor SP responses is more difficult due to both CAP attenuation and superimposition of the prolonged negative component. We identified the SP as the fast negative deflection arising from baseline with peak latencies comparable to mean SP latency in controls. This rapid deflection was followed by the CAP or the prolonged neural activity and showed normal or attenuated amplitude. Figure 6 displays the SP–CAP responses recorded from subject #2 at stimulation intensities from 120 to 100 dB p.e. SPL superimposed on the corresponding curves recorded from one control. The initial fast negative deflection in subject #2 closely followed the profile of the SP in the control. Since SP is acknowledged to be primarily generated by IHCs at high stimulation intensities (Zheng et al. 1997; Durrant et al. 1998; Sellick et al. 2003), our findings may indicate that pre-synaptic events arising in IHCs are normal, at least in patients with normal SP amplitude.
Comparison between the SP–CAP potentials recorded from one subject with OTOF mutations (#2, right ear) and one control. The curves displayed in Figure 2 for subject #2 are superimposed on the recordings obtained from one control at intensities from 120 to 100 dB p.e. SPL to highlight the similarities of the SP component between controls and patients with OTOF mutations. Note that the initial fast negative deflection recorded from subject #2 closely followed the profile of SP in the control recordings. Filled circles and triangles refer to the CAP and SP peaks, respectively.
A small number of subjects were included in this study. The reason was twofold. First of all, OTOF mutations have a low incidence in children affected by pre-lingual profound hearing loss (Rodríguez-Ballesteros et al. 2008). Moreover, transtympanic ECochG, an invasive procedure, is usually performed as part of the audiological evaluation preceding cochlear implantation. Nevertheless, in spite of the small number of children included in this study, the findings we obtained are reasonably consistent across subjects, especially if one accepts the argument that a prolonged SP–CAP complex represents a less-severe disorder than a normal SP with no evidence of a CAP.
The OTOF gene contains 49 exons and encodes multiple long and short isoforms of the protein, by alternative splicing combined with the use of several translation initiation sites (Yasunaga et al. 2000). The first 19 exons are exclusive of the long isoforms. In previous studies, AN has been observed in subjects carrying two mutations affecting all otoferlin isoforms or one mutation affecting all isoforms and the other affecting only the long isoforms (Rodríguez-Ballesteros et al. 2008 for review). This work is the first to report one patient (subject #2) carrying two truncating mutations affecting only the long isoforms and presenting with auditory neuropathy. Although this means that any combination of mutations in the OTOF gene results in auditory neuropathy, it is tempting to speculate that the subtle differences in ECochG recordings found amongst patients could be partly related to the nature (truncating, non-truncating) and location (long isoforms, all isoforms) of their mutations.
This is the first study reporting the features of cochlear potentials in human subjects with OTOF mutations. Experimental models, whilst contributing to elucidating the mechanisms underlying the functional alterations in the auditory periphery, cannot exactly reproduce the human phenotype.
References
Dallos P, Wang CY. Bioelectric correlates of kanamycin intoxication. Audiology 13:277–289, 1974.
Durrant JD, Wang J, Ding DL, Salvi RJ. Are inner or outer hair cells the source of summating potentials recorded from the round window? J. Acoust. Soc. Am. 104:370–377, 1998.
Eggermont JJ. Electrocochleography. In: Keidel WD and Neff WD (eds) Handbook of sensory physiology. Auditory system. Springer, New York, pp. 625–705, 1976.
Eggermont JJ, Odenthal DW. Methods in electrocochleography. Acta Otolaryngol. Suppl. 316:17–24, 1974a.
Eggermont JJ, Odenthal DW. Action potentials and summating potentials in the normal human cochlea. Acta Otolaryngol. Suppl. 316:39–61, 1974b.
Eggermont JJ, Ponton CW, Coupland SG, Winkelaar R. Maturation of the traveling-wave delay in the human cochlea. J. Acoust. Soc. Am. 90:288–298, 1991.
European Concerted Action Project on Genetics of Hearing impairment. Study group on terminology, definition and hearing assessment. Newsletter 1996.
Ferraro JA, Thedinger BS, Mediavilla SJ, Blackwell WL. Human summating potential to tone bursts: observations on tympanic membrane versus promontory recordings in the same patients. J. Am. Acad. Audiol. 5:24–29, 1994.
Jiménez JL, Bashir R. In silico functional and structural characterisation of ferlin proteins by mapping disease-causing mutations and evolutionary information onto three-dimensional models of their C2 domains. J. Neurol. Sci. 260:114–123, 2007.
McMahon CM, Patuzzi RB, Gibson WP, Sanli H. Frequency-specific electrocochleography indicates that presynaptic and postsynaptic mechanisms of auditory neuropathy exist. Ear Hear. 29:314–325, 2008.
Migliosi V, Modamio-Hoybjor S, Moreno-Pelayo MA, Rodriguez-Ballesteros M, Villamar M, Tellería D, Menéndez I, Moreno F, Del Castillo I. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non-syndromic hearing loss. J. Med. Genet. 39:502–506, 2002
Petit C, Levilliers J, Hardelin JP. Molecular genetics of hearing loss. Annu. Rev. Genet. 35:589–646, 2001.
Prieve BA, Fitzgerald TS, Schulte LE. Basic characteristics of click-evoked otoacoustic emissions in infants and children. J. Acoust. Soc. Am. 102:2860–2870, 1997.
Rapin I, Gravel J. “Auditory neuropathy”: physiologic and pathologic evidence calls for more diagnostic specificity. Int. J. Pediatr. Otorhinolaryngol. 67:707–728, 2003.
Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 273:15879–15882, 1998.
Rodriguez-Ballesteros M, del Castillo FJ, Martin Y, Moreno-Pelayo MA, Morera C, Prieto F, Marco J, Morant A, Gallo-Terán J, Morales-Angulo C, Navas C, Trinidad G, Tapia MC, Moreno F, del Castillo I. Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF). Hum. Mutat. 22:451–456, 2003.
Rodríguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, Arslan E, Medá C, Curet C, Völter C, Sainz-Quevedo M, Castorina P, Ambrosetti U, Berrettini S, Frei K, Tedín S, Smith J, Cruz Tapia M, Cavallé L, Gelvez N, Primignani P, Gómez-Rosas E, Martín M, Moreno-Pelayo MA, Tamayo M, Moreno-Barral J, Moreno F, del Castillo I. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with non-syndromic hearing impairment and auditory neuropathy. Hum. Mutat. 29:823–831, 2008.
Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127:277–289, 2006.
Santarelli R, Arslan E. Electrocochelography in auditory neuropathy. Hear. Res. 170:32–47, 2002.
Santarelli R, Scimemi P, Dal Monte E, Arslan E. Cochlear microphonic potential recorded by transtympanic electrocochleography in normally-hearing and hearing-impaired ears. Acta Otorhinolaryngol. Ital. 26:78–95, 2006.
Santarelli R, Starr A, Michalewski H, Arslan E. Neural and receptor cochlear potentials obtained by transtympanic electrocochleography in auditory neuropathy. Clin. Neurophysiol. 119:1028–1041, 2008.
Schmidt PH, Eggermont JJ, Odenthal DW. Study of menière’s disease by electrocochleography. Acta Otolaryngol. Suppl. 316:75–84, 1974.
Sellick P, Patuzzi R, Robertson D. Primary afferents and cochlear nerve contribution to extracellular potentials during tone-bursts. Hear. Res. 176:42–58, 2003.
Starr A. The neurology of auditory neuropathy. In: Sininger Y and Starr A (eds) Auditory neuropathy: a new perspective on hearing disorders. Singular, San Diego, pp. 37–51, 2001.
Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain 119:741–753, 1996.
Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J. Basic Clin. Physiol. Pharmacol. 11:215–230, 2000.
Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear Hear. 22:91–99, 2001.
Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser). Brain 126:1604–1619, 2003.
Starr A, Isaacson B, Michalewski HJ, Zeng FG, Kong YY, Beale P, Paulson GW, Keats BJ, Lesperance MM. A dominantly inherited progressive deafness affecting distal auditory nerve and hair cells. J. Assoc. Res. Otolaryngol. 5:411–426, 2004.
Withnell RH. Brief report: the cochlear microphonic as an indication of outer hair cell function. Ear Hear. 22:75–77, 2001.
Yasunaga S, Grati M, Chardenoux S, Smith TN, Friedman TB, Lalwani AK, Wilcox ER, Petit C. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am. J. Hum. Genet. 67:591–600, 2000.
Zheng XY, Ding DL, McFadden SL, Henderson D. Evidence that inner hair cells are the major source of cochlear summating potentials. Hear. Res. 113:76–88, 1997.
Acknowledgments
We are grateful to Dr. Erica Dal Monte for her invaluable help in data collection, and Mrs. Tiziana Gavagnin and Mrs. Stefania Benetti who collected the blood samples. This work was supported in part by grants to Arnold Starr from the National Institute of Deafness and Other Communication Disorders # DC-02618 and from the European Commission (FP6 Integrated Project EUROHEAR, LSHG-CT-2004-512063), Spanish Ministerio de Educación y Ciencia (SAF2005-06355), Fundacion Mutua Madrileña and Programa “Ramon y Cajal” (to I.d.C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santarelli, R., del Castillo, I., Rodríguez-Ballesteros, M. et al. Abnormal Cochlear Potentials from Deaf Patients with Mutations in the Otoferlin Gene. JARO 10, 545–556 (2009). https://doi.org/10.1007/s10162-009-0181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-009-0181-z