Abstract
Background
In April 2015, five types of phosphate binders (PBs) were available by prescription in Japan, namely calcium carbonate, sevelamer hydrochloride, lanthanum carbonate, bixalomer, and ferric citrate hydrate (FeC). FeC reduces serum phosphorus levels and increases the body’s iron stores. However, it is unclear whether FeC lowers serum phosphorus relative to other agents in a regional practical setting.
Methods
We performed a retrospective observational cohort study of regional hemodialysis surveillance in the western Saitama area of Japan, which included 1374 hemodialysis patients enrolled from 32 satellite dialysis units. The clinical data and prescribing information were retrospectively collected and analyzed. The difference in serum phosphorus among the groups administered five types of PBs (new or additional) from April to September 2015 was the primary outcome.
Results
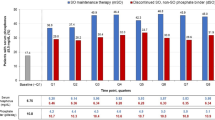
As of April 2015, the median values of serum phosphorus, corrected calcium, and intact parathyroid hormone were 5.4 mg/dL, 9.1 mg/dL, and 147 pg/dL, respectively (N = 1374). Unexpectedly, with an increase in the number of PBs administered, serum phosphorous levels increased (p < 0.001). The significant changes in the serum phosphorus and hemoglobin levels were associated with the prescription of FeC but not with that of the other PBs.
Conclusions
This regional survey suggests that serum phosphorus is well managed and that FeC has the potential to reduce the serum phosphorus level relative to other PBs and to ameliorates anemia.




Similar content being viewed by others
Change history
25 September 2019
In the Original publication, Under the table 1, the number of participants in the April has been incorrectly published as 1373. The corrected table is given below.
References
Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11(3):340–8. https://doi.org/10.1111/j.1542-4758.2007.00190.x.
Danese MD, Belozeroff V, Smirnakis K, Rothman KJ. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(5):1423–9. https://doi.org/10.2215/cjn.01060308.
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann Intern Med. 2018. https://doi.org/10.7326/m17-2640.
Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–88. https://doi.org/10.1111/1744-9987.12058.
Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96. https://doi.org/10.2215/cjn.00290109.
Hammer J, Oesterreicher C, Hammer K, Koch U, Traindl O, Kovarik J. Chronic gastrointestinal symptoms in hemodialysis patients. Wien Klin Wochenschr. 1998;110(8):287–91.
Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. https://doi.org/10.1053/j.ackd.2006.10.001.
Gebretsadik Z, Gebrehans M, Getnet D, Gebrie D, Alema T, Belay YB. Assessment of drug-drug interaction in ayder comprehensive specialized Hospital, Mekelle, Northern Ethiopia: a retrospective study. Biomed Res Int. 2017;2017:9792363. https://doi.org/10.1155/2017/9792363.
Yokoyama K, Fukagawa M, Akiba T, Nakayama M, Otoguro T, Yamada K, et al. Ferritin elevation and improved responsiveness to erythropoiesis-stimulating agents in patients on ferric citrate hydrate. Kidney Int Rep. 2017;2(3):359–65. https://doi.org/10.1016/j.ekir.2016.12.005.
Umanath K, Jalal DI, Greco BA, Umeukeje EM, Reisin E, Manley J, et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26(10):2578–87. https://doi.org/10.1681/ASN.2014080842.
Palmer SC, Gardner S, Tonelli M, Mavridis D, Johnson DW, Craig JC, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis. 2016;68(5):691–702. https://doi.org/10.1053/j.ajkd.2016.05.015.
Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. https://doi.org/10.1681/asn.2014020212.
Panichi V, Rosati A, Bigazzi R, Paoletti S, Mantuano E, Beati S, et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26(8):2641–8. https://doi.org/10.1093/ndt/gfq802.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. https://doi.org/10.1038/bmt.2012.244.
Slatopolsky EA, Burke SK, Dillon MA. RenaGel, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. The RenaGel Study Group. Kidney Int. 1999;55(1):299–307. https://doi.org/10.1046/j.1523-1755.1999.00240.x.
D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl. 2003(85):S73-8. https://doi.org/10.1046/j.1523-1755.63.s85.18.x.
Slatopolsky E, Weerts C, Lopez-Hilker S, Norwood K, Zink M, Windus D, et al. Calcium carbonate as a phosphate binder in patients with chronic renal failure undergoing dialysis. N Engl J Med. 1986;315(3):157–61. https://doi.org/10.1056/nejm198607173150304.
Akizawa T, Origasa H, Kameoka C, Kaneko Y, Kawasaki S, Bixalomer Study G. Randomized controlled trial of bixalomer versus sevelamer hydrochloride in hemodialysis patients with hyperphosphatemia. Ther Apher Dial. 2014;18(2):122–31. https://doi.org/10.1111/1744-9987.12068.
Fissell RB, Karaboyas A, Bieber BA, Sen A, Li Y, Lopes AA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial Int. 2016;20(1):38–49. https://doi.org/10.1111/hdi.12315.
Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. 2014;29(11):2092–9. https://doi.org/10.1093/ndt/gft280.
Martins MT, Silva LF, Kraychete A, Reis D, Dias L, Schnitman G, et al. Potentially modifiable factors associated with non-adherence to phosphate binder use in patients on hemodialysis. BMC Nephrol. 2013;14:208. https://doi.org/10.1186/1471-2369-14-208.
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–83. https://doi.org/10.1056/nejm200005183422003.
Komaba H, Wang M, Taniguchi M, Yamamoto S, Nomura T, Schaubel DE, et al. Initiation of Sevelamer and Mortality among Hemodialysis Patients Treated with Calcium-Based Phosphate Binders. Clin J Am Soc Nephrol. 2017;12(9):1489–97. https://doi.org/10.2215/cjn.13091216.
Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–52. https://doi.org/10.1046/j.1523-1755.2002.00434.x.
Tsuchida K, Nagai K, Yokota N, Minakuchi J, Kawashima S. Impact of lanthanum carbonate on prognosis of chronic hemodialysis patients: a retrospective cohort study (Kawashima Study). Ther Apher Dial. 2016;20(2):142–8. https://doi.org/10.1111/1744-9987.12399.
Shigematsu T, Tokumoto A, Nakaoka A, Arisaka H. Effect of lanthanum carbonate treatment on bone in Japanese dialysis patients with hyperphosphatemia. Ther Apher Dial. 2011;15(2):176–84. https://doi.org/10.1111/j.1744-9987.2010.00898.x.
Acknowledgements
The authors acknowledge all hemodialysis facilities participated: Wakaba Internal Clinic, Ome Kidney Clinic, Musashimurayama Hospital, Koujinkai Takasaka Medical Clinic, Gyoda General Hospital, Ikebukuro Hospital, Ogose Medical Clinic, Kanetsu Hospital, Matsumoto Clinic, Kobayashi Internal Clinic, Tsurugashima Ekimae Clinic, Oka Hospital, Higashihanno Ekimae Clinic, Kamino Clinic, Kokusaiji Clinic, Yoriihoncyo Clinic, Sakura Memorial Hospital, Shalom Hospital, Gyoda Central General Hospital, Higashimatsuyama Kojin Clinic, Koujinkai Ogawa Hospital, Chichibu Municipal hospital, Okamura Memorial Hospital, Chichibu Daiichi Hospital, Musashidai Hospital, Minano Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The contributing authors reported the following financial supports: Hirokazu Okada received Astellas Pharma Inc, Takeda Pharmacological Company, Pfizer Japan Inc, MSD Inc, Novartis Pharma Japan, Otsuka Pharmacological Inc, Kyowa Hakko Kirin, Daiichi Sankyo Inc, Mitsubishi Tanabe Parma, Cyugai Pharma, Torii Pharma, Bayer Yakuhin, Boehringer-Ingelheim Japan, and Eli Lilly Japan.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 16–023) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
We obtained consent through the opt-out procedure from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Amano, H., Ohno, Y., Inoue, T. et al. Regional prescription surveillance of phosphate binders in the western Saitama area: the substantial role of ferric citrate hydrate in improving serum phosphorus levels and erythropoiesis. Clin Exp Nephrol 23, 841–851 (2019). https://doi.org/10.1007/s10157-019-01715-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01715-8