Abstract
Background
Renal hypouricemia is a rare heterogeneous inherited disorder characterized by impaired tubular uric acid transport with severe complications, such as acute kidney injury and nephrolithiasis. Type 1 is caused by a loss-of-function mutation in the SLC22A12 gene (URAT1), while type 2 is caused by defects in the SLC2A9 gene (GLUT9).
Methods and results
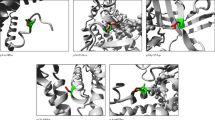
In this article we present clinical, biochemical and molecular genetics of two Czech patients. The serum uric acid in the probands was 57 and 98 µmol/l and expressed as an increase in the fractional excretion of uric acid (40 and 18 %). The sequencing analysis of SLC22A12 and SLC2A9 revealed novel variants p.R92C and p.R203C in URAT1 and p.G72D in GLUT9. Functional studies were performed for these novel variants and for previously reported variants p.I118HfsX27, p.G216R and p.N333S in GLUT9 responsible for renal hypouricemia in three probands from Czech Republic and United Kingdom. Functional studies showed significantly decreased urate uptake for all variants. However, urate uptake of GLUT9 variants prepared for both isoforms were not significantly different.
Conclusions
This is the first complex function characterization of non-synonymous allelic variants in patients with renal hypouricemia regarding both GLUT9 isoforms. Our finding of defects in the SLC2A9 and SLC22A12 genes show the following: renal hypouricemia is not restricted to East Asia populations; urate uptake of GLUT9 variants prepared for both isoforms were not significantly different; renal hypouricemia type 2 has more wide clinical variability than type 1; the phenotypic severity of renal hypouricemia is not correlated with results of functional characterizations of URAT1 and GLUT9 variants.

Similar content being viewed by others
References
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52.
Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics. 2000;66:217–20.
Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–73.
Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004;66:935–44.
Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, Hosoya T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet. 2008;74:243–51.
Taniguchi A, Urano W, Yamanaka M, Yamanaka H, Hosoyamada M, Endou H, Kamatani N. A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum. 2005;52:2576–7.
Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, Nakamura T, Morimoto Y, Kamakura K, Sakurai Y, Nonoyama S, Kanai Y, Shinomiya N. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83:744–51.
Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283:26834–8.
Dinour D, Gray NK, Campbell S, Shu X, Sawyer L, Richardson W, Rechavi G, Amariglio N, Ganon L, Sela BA, Bahat H, Goldman M, Weissgarten J, Millar MR, Wright AF, Holtzman EJ. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21:64–72.
Stiburkova B, Ichida K, Sebesta I. Novel homozygous insertion in SLC2A9 gene caused renal hypouricemia. Mol Genet Metab. 2011;102:430–5.
Shima Y, Nozu K, Nozu Y, Togawa H, Kaito H, Matsuo M, Iijima K, Nakanishi K, Yoshikawa N. Recurrent EIARF and PRES with severe renal hypouricemia by compound heterozygous SLC2A9 mutation. Pediatrics. 2011;127:e1621–5.
Stiburkova B, Taylor J, Marinaki AM, Sebesta I. Acute kidney injury in two children caused by renal hypouricaemia type 2. Pediatr Nephrol. 2012;27:1411–5.
Dinour D, Bahn A, Ganon L, Ron R, Geifman-Holtzman O, Knecht A, Gafter U, Rachamimov R, Sela BA, Burckhardt G, Holtzman EJ. URAT1 mutations cause renal hypouricemia type 1 in Iraqi Jews. Nephrol Dial Transplant. 2011;26:2175–81.
Tasic V, Hynes AM, Kitamura K, Cheong HI, Lozanovski VJ, Gucev Z, Jutabha P, Anzai N, Sayer JA. Clinical and functional characterization of URAT1 variants. PLoS One. 2011;6:e28641.
Stiburkova B, Sebesta I, Ichida K, Nakamura M, Hulkova H, Krylov V, Kryspinova L, Jahnova H. Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: biochemical, genetics and functional analysis. Eur J Hum Genet. 2013;21:1067–73.
Bhasin B, Stiburkova B, De Castro-Pretelt M, Beck N, Bodurtha JN, Atta MG. Hereditary renal hypouricemia: a new role for allopurinol? Am J Med. 2014;127:e3–4.
Sebesta I, Stiburkova B, Bartl J, Ichida K, Hosoyamada M, Taylor J, Marinaki A. Diagnostic tests for primary renal hypouricemia. Nucleosides Nucleotides Nucleic Acids. 2011;30:1112–6.
Stiburkova B, Krijt J, Vyletal P, Bartl J, Gerhatova E, Korinek M, Sebesta I. Novel mutations in xanthine dehydrogenase/oxidase cause severe hypouricemia: biochemical and molecular genetic analysis in two Czech families with xanthinuria type I. Clin Chim Acta. 2012;413:93–9.
Hurba O, Mancikova A, Krylov V, Pavlikova M, Pavelka K, Stiburkova B. Complex analysis of urate transporters SLC2A9, SLC22A12 and functional characterization of non-synonymous allelic variants of GLUT9 in the Czech population: no evidence of effect on hyperuricemia and gout. PLoS One. 2014;9:e107902.
Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61.
Stiburkova B, Zavada J, Tomcik M, Miyata H, Toyoda Y, Takada T, Suzuki H. Novel dysfunctional variant in ABCG2 gene is a cause of primary hyperuricemia and gout: biochemical, molecular genetic and functional analysis. Rheumatology. 2015; doi:10.1093/rheumatology/kev3507.
Stiburkova B, Stekrova J, Nakamura M, Ichida K. Hereditary renal hypouricemia type 1 and autosomal dominant polycystic kidney disease. Am J Med Sci. 2015;350:268–71.
Kimura T, Takahashi M, Yan K, Sakurai H. Expression of SLC2A9 isoforms in the kidney and their localization in polarized epithelial cells. PLoS One. 2014;9:e84996.
Witkowska K, Smith KM, Yao SY, Ng AM, O’Neill D, Karpinski E, Young JD, Cheeseman CI. Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses. Am J Physiol Renal Physiol. 2012;303:F527–39.
Karns R, Zhang G, Sun G, Indugula SR, Cheng H, Havas-Augustin D, Novokmet N, Rudan D, Durakovic Z, Missoni S, Chakraborty R, Rudan P, Deka R. Genomewide association of serum uric acid concentration: replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann Hum Genet. 2012;76:121–7.
Gabrikova D, Bernasovska J, Sokolova J, Stiburkova B. High frequency of SLC22A12 variants causing renal hypouricemia 1 in the Czech and Slovak Roma population; simple and rapid detection method by allele-specific polymerase chain reaction. Urolithiasis. 2015;43:441–5.
Diaz-Torne C, Perez-Herrero N, Perez-Rui F. New medications in development for the treatment of hyperuricemia of gout. Curr Opin Rheumatol. 2015;27:164–9.
Jeannin G, Chiarelli N, Gaggiotti M, Ritelli M, Maiorca P, Quinzani S, Verzeletti F, Possenti S, Colombi M. Cancarini G. Recurrent exercise-induced acute renal failure in a young Pakistani man with severe renal hypouricemia and SLC2A9 compound heterozygosity. BMC Med Genet. 2014;15:3.
Acknowledgments
Supported by grants from the Czech Republic: Ministry of Education, Youth and Sports (LH13245), by a project from the Ministry of Health for conceptual development of research organization 00023728 (Institute of Rheumatology) and by institutional support from a program at Charles University in Prague (PRVOUK-P24/LF1/3, SVV UK 260148/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Mancikova, A., Krylov, V., Hurba, O. et al. Functional analysis of novel allelic variants in URAT1 and GLUT9 causing renal hypouricemia type 1 and 2. Clin Exp Nephrol 20, 578–584 (2016). https://doi.org/10.1007/s10157-015-1186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1186-z