Abstract
Background
Gangliosides are amphipathic lipids ubiquitously expressed in all vertebrate cells. They have been reported to play pivotal roles in cell morphology, cell adhesion, signal transduction, and modulation of immune reaction. Although human kidney contains various kinds of ganglioside, their physiological and pathophysiological roles have not been elucidated yet. As ganglioside GM3 is the most abundant ganglioside in human kidney, we tried to reveal the distribution of GM3 using histological analysis.
Methods
Macroscopically normal parts of operatively resected kidney from renal cell carcinoma patients were used for analyses. Immunohistochemical and immunoelectron microscopic analyses were performed with anti-GM3 antibody.
Results
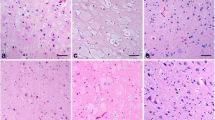
Immunohistochemical analyses showed that GM3 was observed in glomeruli and renal proximal tubules. Immunoelectron microscopy demonstrated that GM3 was localized on the foot process of podocyte and also in Golgi region of renal proximal tubule cells.
Conclusions
Ganglioside GM3 might take a part of the negative electric charge on the surface of podocyte and its multiple physiological actions may play pivotal roles for maintaining glomerular function.





Similar content being viewed by others
References
Hakomori S. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochem Biophys Acta. 2008;1780:325–46.
Todeschini AR, Hakomori S. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth through glycosynaptic microdomains. Biochem Biophys Acta. 2008;1780:421–33.
Yu RK, Tsai YT, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides-an overview. J Oleo Sci. 2011;60:537–44.
Chaves DEP, Sipione S. Sphingolipids and gangliosides of nervous system in membrane function and dysfunction. FEBS Lett. 2009;584:1748–59.
Kojima N, Kurosawa N, Nishi T, Hanai N, Tsuji S. Induction of cholinergic differentiation with neurite sprouting by de novo biosynthesis and expression of GD3 and b-series gangliosides in neuro2a cells. J Biol Chem. 1994;269:30451–6.
Iwabuchi K, Yamamura S, Prinetti A, Handa K, Hakomori S. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate–carbohydrate interaction in mouse melanoma B16 cells. J Biol Chem. 1998;273:9130–8.
Kanda N, Tamaki K. Ganglioside GT1b suppresses immunoglobulin production by human peripheral blood mononuclear cells. Immunology. 1999;96:628–33.
Mukherjee P, Faber AC, Shelton LM, Baek RC, Chiles TC, Seyfied TN. Ganglioside GM3 suppresses the pro-angiogenic effects of vascular endothelial growth factor and ganglioside GD1a. J Lipid Res. 2008;49:929–38.
Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K, Furukawa K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance. Proc Natl Acad Sci USA. 2009;106:1–6.
Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Aca Sci USA. 2007;104:13678–83.
Kikkawa Y, Mimura A, Inage Z. Regional distribution of sulfatide in human kidney, and anti-sulfatide antibodies in sera from patients with nephritis detected by TLC immunostaining. Jpn J Nephrol. 1991;33:635–42.
Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976;251:7517–20.
Jing J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–99.
Inokuchi J. Physiopathological function of hematoside (GM3 ganglioside). Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:179–98.
Holthofer H, Reivinen J, Miettinen A. Nephron segment and cell-type specific expression of gangliosides in the developing and adult kidney. Kidney Int. 1994;45:123–30.
Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of n-glycolylneuraminic acid in Humans. J Biol Chem. 1998;273:15866–71.
Kanai Y, Kawakami H, Takata K, Kurohmura M, Hayashi Y, Nishida T, Hirano H. Localization of Forssman glycolipid and GM1 ganglioside intracellularly and on the surface of germ cells during fetal testicular and ovarian development of mice. Histochemistry. 1990;94:561–8.
Hotta T, Kawakami H, Fukuda M, Yoshino Y, Hirano H. Detection of disialoganglioside in rat cerebellar cortex by light and electron microscopy. Acta Histochem Cytochem. 2000;33:281–5.
Taki T, Handa S, Ishikawa D. Blotting of glycolipids and phospholipids from a high-performance thin-layer chromatogram to a polyvinylidene difluoride membrane. Anal Biochem. 1994;221:312–6.
Kaneko T, Moriyama T, Imai E, Akagi Y, Arai M, Inoue T, Xia C, Noguchi T, Kamada T, Ueda N. Expression of transmembrane-type protein tyrosine phosphatase mRNA along rat nephron segments. Am J Physiol Ren Physiol. 1995;268:F1102–8.
Hirabayashi Y, Hamaoka A, Matsumoto M, Matsubara T, Tagawa M, Wakabayashi S, Taniguchi M. Syngeneic monoclonal antibody against melanoma antigen with interspecies cross-reactivity recognizes GM3, a potent ganglioside of B16 melanoma. J Biol Chem. 1985;260:13328–33.
Itonori S, Hidara K, Sanai Y, Taniguchi M, Nagai Y. Involvement of the acyl chain of ceramide in carbohydrate recognition by an anti-glycolipid monoclonal antibody: the case of an anti-melanoma antibody, M2590, to GM3-ganglioside. Glycoconj J. 1989;6:551–60.
Maccioni HJF. Glycosylation of glycolipids in the Golgi complex. J Neurochem. 2007;103(Suppl 1):81–90.
Novak A, Muzinic NR, Culic VC, Bozic J, Kurir TT, Ferhatovic L, Puljak L, Markotic A. Renal distribution of ganglioside GM3 in rat models of types 1 and 2 diabetes. J Physiol Biochem. 2013;69:727–35.
Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91:797–803.
Spiegel S, Matyas GR, Cheng L, Sacktor B. Asymmetric distribution of gangliosides in rat renal brush-border and basolateral membranes. Biochim Biophys Acta. 1988;938:270–8.
Chung TW, Kim SJ, Choi HJ, Kim KJ, Kim MJ, Kim SH, Lee HJ, Ko JH, Lee YC, Suzuki A, Kim CH. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: direct interaction of GM3 with VEGFR-2. Glycobiology. 2009;19:229–39.
Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin––the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591–6.
Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301.
Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez-A C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–7.
Akiyoshi K, Itaya A, Nomura SM, Ono N, Yoshikawa K. Induction of neuron-like tubes and liposome networks by cooperative effect of ganglioside and phospholipids. FEBS Lett. 2003;534:33–8.
Cantu L, Corti M, Brocca P, Favero DE. Structural aspects of ganglioside-containing membranes. Biochim Biophys Acta. 2009;1788:202–8.
Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthofer H. Involvement of lipid rafts in nephrin phosphorylation and organization of glomerular slit diaphragm. Am J Pathol. 2001;159:1069–77.
Crew VK, Burton N, Kagen A, Green CA, Levene C, Flinter F, Brady RL, Daniels G, Anstee DJ. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 2004;104:2217–23.
Acknowledgments
We thank Ms. Sachie Matubara (Laboratory for Electron microscopy, Kyorin University School of Medicine) for electron microscopic analyses. We also thank Dr. Takao Taki (Tokushima Institute, Otsuka Pharmaceutial Co., Ltd), for providing control samples of ganglioside, technical advice and helpful discussion.
Conflict of interest
Potential financial conflict of interest.
Honoraria: Yoshiharu Tsubakihara (Chugai Pharm Co., Ltd, Kyowa Hakko, Kirin Co.,Ltd, Mitsubishi Tanabe Co., Ltd, Bayer Co., Ltd,), Research funding: Yoshiharu Tsubakihara (Chugai Pharm Co., Ltd, Baxter Japan Ltd, Otsuka Pharm Co., Ltd, Bayer Co., Ltd,), Endowed departments by commercial entities: Yoshiharu Tsubakihara (Chugai Pharm Co., Ltd, Baxter Japan Ltd,). The other authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kaneko, T., Tsubakihara, Y., Fushimi, H. et al. Histochemical and immunoelectron microscopic analysis of ganglioside GM3 in human kidney. Clin Exp Nephrol 19, 403–410 (2015). https://doi.org/10.1007/s10157-014-1003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-014-1003-0