Abstract
Dyslipidemia is an independent risk factor for the development and progression of diabetic nephropathy (DN). In this review, we summarize mouse models with both diabetes and dyslipidemia, and their associated complications. We then discuss molecules potentially involved in deterioration of DN by dyslipidemia. We focus especially upon toll-like receptor 4 (TLR4) and one of its endogenous ligands, myeloid-related protein 8 (MRP8 or S100A8), since we have found that their mRNA levels are commonly increased in glomeruli of type 1 (streptozotocin [STZ]-induced) and type 2 (A-ZIP/F-1 lipoatrophic) diabetic mice. Gene expression of MRP8 and Tlr4 is further upregulated during worsening of STZ-induced DN by a high fat diet (HFD). Moreover, these HFD-induced changes are accompanied by enhanced gene expression of CCAAT element binding protein β and phosphorylation of c-Jun N-terminal kinase in the kidney, which have also been reported in pancreatic β cells under diabetic-hyperlipidemic conditions. Effects of a HFD upon DN are cancelled in Tlr4 knockout mice. Macrophages are the predominant source of MRP8 in glomeruli. In cultured macrophages, combinatorial treatment with high glucose and palmitate amplifies MRP8 expression in a Tlr4-dependent manner, and recombinant MRP8 protein markedly increases gene expression of the inflammatory cytokines interleukin-1β and tumor necrosis factor α. Here, we propose ‘macrophage-mediated glucolipotoxicity’ via activation of MRP8/TLR4 signaling as a novel mechanism of pathophysiology for DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since only one-third of patients with type 1 diabetes develop diabetic nephropathy (DN), we should consider the role of factors other than hyperglycemia in the pathophysiology of DN, including genetic, epigenetic, environmental and metabolic aspects. Several reports describe hyperlipidemia or dyslipidemia as an independent risk factor for the progression of DN in type 1 and type 2 diabetes, as well as for atherosclerotic complications [1–4]. Using type 1 (streptozotocin [STZ]-induced) and type 2 (db/db) diabetic mouse models, we have confirmed that treatment of diabetic mice with a high fat diet (HFD) exacerbates albuminuria and glomerular lesions [5]. Of note, single nucleotide polymorphisms in acetyl-CoA carboxylase β gene, which plays an important role in the regulation of fatty acid metabolism, exhibit a potent association with proteinuria in patients with type 2 diabetes [6, 7]. Accordingly, a concept of synergistic toxicity caused by glucose and lipid, described as ‘glucolipotoxicity’, has emerged in recent years. However, the underlying molecular mechanism is still obscure, especially in renal complication [8]. Here we will discuss diabetic-hyperlipidemic mouse models and glucolipotoxicity in the kidney.
Diabetic-hyperlipidemic mouse models
As described above, several clinical and experimental phenomena have highlighted the synergistic effects of hyperglycemia and hyperlipidemia upon the development and progression of diabetic complications including nephropathy. Despite the fact that there are several limitations associated with the difference in hyperlipidemia between rodents and humans, mouse models are still most widely used to study complications caused by diabetes and hyperlipidemia. The reasons include small animal size, short generation time, the ease of induction of diabetes, hyperlipidemia or gene manipulation, and cost effectiveness [9]. Hence, in the last decade diabetic-hyperlipidemic mouse models have been used for genetic modification, pharmacological treatment and/or some particular chow diets that abundantly contain fat and/or cholesterol. In this section, representative mouse models are summarized.
Apolipoprotein E-deficient mice treated with streptozotocin (ApoE KO + STZ)
ApoE KO + STZ mice are one of the most popular diabetic-hyperlipidemic mouse models. This model shows not only hypercholesterolemia and hypertriglyceridemia, but also accelerated aortic atherosclerotic lesions [10–12] and nephropathy [13–15] associated with diabetes. These reports revealed that advanced glycation end-products [13, 14] and endoplasmic reticulum (ER) stress [16, 17] are candidate mediators of glucolipotoxicity in ApoE KO + STZ mice.
Low-density lipoprotein (LDL) receptor-deficient mice treated with STZ (LDLR KO + STZ)
LDLR KO + STZ mice show dyslipidemia including high LDL cholesterol, low high-density lipoprotein (HDL) cholesterol levels and hypertriglyceridemia, mimicking human metabolic syndrome [18]. Moreover, addition of a HFD exacerbates hypertriglyceridemia, hypercholesterolemia, and diabetic renal lesions (including glomerular and tubulointerstitial macrophage infiltration) in this model [19]. The authors [19] referred to an earlier work indicating that irradiation-induced depletion of bone marrow cells (including monocytes) reduces renal injury in STZ-diabetic rats [20].
STZ-induced diabetic mice with HFD feeding (STZ + HFD)
A supplemental HFD on STZ-treated diabetic mice increases blood triglyceride and free fatty acid concentrations, at least in part, because of insulin deficiency, suggesting that this model might be useful especially for analyzing pathophysiology by high triglyceride-rich lipoprotein and/or high free fatty acids coexisting with high glucose conditions. In STZ + HFD mice, there are several reports describing vascular complications such as cardiovascular dysfunction [21], retinopathy [22], neuropathy [23] and nephropathy [5, 24].
Treatment of wild-type mice with STZ and HFD synergistically increases albuminuria [5] and expands mesangial area (Fig. 1). Induction of diabetes by STZ causes a marked increase in urine volume and creatinine clearance of normal diet-fed and HFD-fed animals, respectively, suggesting that glomerular hyperfiltration has occurred. On the other hand, HFD treatment reduces urine volume and creatinine clearance in STZ mice (Fig. 1), suggesting that HFD is not causing more hyperfiltration but is causing non-hemodynamic actions which will be discussed below.
Effects of STZ and/or HFD upon mesangial expansion (a), urine volume (b) and creatinine clearance (c) in wild-type mice. nSTZ-ND non STZ-normal diet, nSTZ-HFD non STZ-high fat diet, STZ-ND STZ-normal diet, STZ-HFD STZ-high fat diet. Data are mean ± SEM. n = 4–11. *p < 0.01, **p < 0.001. Modified from Kuwabara and others [5]
A-ZIP/F-1 lipoatrophic diabetic mice
A-ZIP/F-1 mice are a genetic mouse model of lipoatrophic diabetes, characterized by severe insulin resistance, dyslipidemia including hypertriglyceridemia and high free fatty acids, and fatty liver [25, 26]. This model is based upon dominant-negative expression of B-ZIP transcription factors of both C/EBP and Jun families under the control of aP2 enhancer/promoter, causing paucity of adipose tissue. A-ZIP/F-1 mice may serve as a useful tool for studying DN, because they manifest severe nephrotic syndrome and typical histopathological renal lesions which are glomerular hypertrophy, diffuse and pronounced mesangial expansion and accumulation of extracellular matrix [27]. Notably, these renal changes are reversible to some extent by replacement therapy with a fat-derived hormone leptin [27].
Other mouse models
There are a few other diabetic-hyperlipidemic mouse models such as non-obese diabetic mice or Ins2 Akita diabetic mice combined with HFD feeding [28, 29], but their renal involvement has not been characterized well. Regardless of the models described above, differences in genetic backgrounds critically affect glucose and lipid metabolism among mouse strains [30]. Furthermore, even similar levels of hyperglycemia cause distinct renal changes among different strains and species. For instance, the DBA/2 strain is highly susceptible to DN, whereas the C57BL/6 strain is relatively resistant [31–33]. In addition, since cholesteryl ester transfer protein is inactive in rodents, HDL is the dominant lipoprotein in mice [34]. Apolipoprotein B in rodents also differs from that in humans [35].
Molecules involved in glucolipotoxicity in the kidney and pancreatic β cells
Although glucotoxicity and lipotoxicity were originally proposed as independent concepts, Prentki et al. reported a novel concept of glucolipotoxicity in pancreatic β cells in 1996. They reported that elevated ambient levels of glucose and free fatty acid cause synergistic inhibition of insulin secretion [36]. On the other hand, they reported that increased intracellular glucose-derived metabolites inhibit enzymes for β-oxidation, leading to cytosolic accumulation of lipids [37]. Subsequently, there have been several reports about the molecular mechanism underlying glucolipotoxicity involved in pancreatic β cell dysfunction and insulin resistance [38–40]. Furthermore, phenomena of glucolipotoxicity are also observed in DN of humans [1–4] and rodents [41, 42], but their pathophysiology remains largely unknown [8]. Here, we will compare glucolipotoxicity upon pancreatic β cell dysfunction and DN.
c-Jun N-terminal kinase (JNK)
JNK plays a pivotal role in ER stress-induced ‘unfolded protein response’ in innate immune system [43]. It was later revealed that ER stress-induced JNK activation is associated with chronic inflammation or high ambient fatty acid levels in obesity or type 2 diabetes [44, 45]. In pancreatic β-cells, high glucose concentrations augment lipotoxicity through JNK activation, at least partly, in an ER stress-dependent manner [46, 47]. In our diabetic-hyperlipidemic model [5], treatment with STZ and HFD synergistically increases phosphorylation of IκB and mRNA expression of pro-inflammatory genes in the kidney, in parallel with phosphorylation of JNK, but not with phosphorylation of other mitogen-activated protein (MAP) kinases such as p38 or extracellular signal-regulated kinase (ERK) (Fig. 2).
Western blot analysis for phosphorylation of MAP kinases and IκB in kidney of STZ + HFD mice. p-/t-p38 phosphorylated/total p38 MAP kinase, p/tERK phosphorylated/total extracellular signal-regulated kinase, p/tJNK phosphorylated/total c-Jun N-terminal kinase, pIκB phosphorylated inhibitor of κB. Modified from Kuwabara and others [5]
CCAAT element binding protein beta (C/EBPβ)
CCAAT element binding protein beta (C/EBPβ) is one of the transcriptional repressors of insulin gene and induced by chronic hyperglycemia [48]. C/EBPβ is increased by fatty acids through the Per-Arnt-Sim kinase (PASK) pathway [49] in pancreatic β cells. Since PASK is also induced by high glucose conditions, these mechanisms may possibly exert glucolipotoxic effects. In the kidney, C/EBPβ is increased in diabetic rats, but not other C/EBP isoforms [50]. Furthermore, renal upregulation of C/EBPβ mRNA in STZ-induced diabetic mice is further enhanced by additional HFD feeding in our experiments [5].
Of note, JNK/AP-1 and C/EBPβ pathways may also contribute to glucolipotoxicity-induced renal damage through upregulation of myeloid-related protein 8 (MRP8, also known as S100A8 or calgranulin A), whose gene promoter region contains AP-1 binding site [51, 52] and C/EBP motif [53, 54], as discussed in the next section.
Fetuin A
Over the last few years, there has been growing evidence for fatty acid-induced lipotoxicity, such as insulin resistance, through toll-like receptor 4 (TLR4) [55–57]. However, it is still controversial whether fatty acid stimulates TLR4 directly or indirectly. Recently, fetuin A has been identified as an adopter protein combining fatty acids and TLR4 [58], and its plasma levels are elevated in diabetic humans and mice [59, 60]. ER stress induced by high glucose and palmitate increases the expression of fetuin A [60], suggesting that fetuin A could hypothetically participate in glucolipotoxicity upon macrophages.
MRP8/TLR4
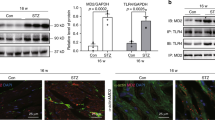
MRP8 was originally identified as a cytoplasmic calcium-binding protein in neutrophils and monocytes [61]. MRP8, by making a heterodimer with MRP14 (or S100A9), has become widely recognized as a potent endogenous ligand for TLR4 in various diseases including septic shock and vascular and autoimmune disorders [62–64]. To identify candidate disease-modifying molecules in DN, we have performed microarray analysis using isolated glomeruli from two different diabetic models of mice—STZ-induced insulin-dependent diabetic mice and lipoatrophic insulin-resistant A-ZIP/F-1 mice. We then focused upon MRP8 and Tlr4, because expression of both genes is commonly increased in these two models [5]. It is noteworthy that diabetic-hyperlipidemic mice such as STZ-HFD mice or A-ZIP/F-1 mice show remarkable upregulation of MRP8 and Tlr4 compared to control non-diabetic mice (Fig. 3). Since macrophages are identified as the major source of MRP8 in the glomeruli of STZ-HFD mice [5], we examined the effects of high glucose and fatty acid on the expression of MRP8 (Fig. 4) and Tlr4 in cultured macrophages. This in vitro study showed that treatment with fatty acid amplifies MRP8 expression only under high ambient glucose conditions. Although Tlr4 is expressed slightly more in high glucose conditions than in low glucose conditions, fatty acid does not alter Tlr4 expression [5]. In addition, synergistic effects with high glucose and fatty acid on macrophages and diabetic kidneys are abrogated by Tlr4 deletion [5] (Fig. 4). Moreover, we have observed that recombinant MRP8 protein markedly increases gene expression of the inflammatory cytokines interleukin-1β and tumor necrosis factor α (TNF-α) in cultured macrophages (submitted) [62]. Similarly, macrophages also play an important role in insulin resistance and β-cell dysfunction through fatty acid-induced TLR4 activation [65, 66]. Particularly in the kidney, MRP8 produced by infiltrated macrophages might exert glucolipotoxic effects upon diabetic glomeruli in a paracrine manner, potentially leading to mesangial expansion, podocyte injury, glomerular sclerosis and albuminuria (Fig. 5), because TLR4 is reportedly expressed in healthy or injured glomerular intrinsic cells including mesangial cells [67, 68], endothelial cells [67, 69] and podocytes [70, 71]. Taken together, we propose ‘macrophage-mediated glucolipotoxicity’ via activation of MRP8/TLR4 signaling as a novel concept for pathophysiology of DN (Fig. 5).
Glomerular gene expression of MRP8 (a) and Tlr4 (b) in STZ + HFD and lipoatrophic A-ZIP/F-1 mice determined by TaqMan real-time PCR. White bars non-diabetic control group, striped bars diabetic group, black bars diabetic-hyperlipidemic group. Data are mean ± SEM. n = 4–7. *p < 0.01, **p < 0.001. Modified from Kuwabara and others [5]
Gene expression of MRP8 and effects of glucose or fatty acid in bone marrow-derived macrophages (BMDMs) determined by TaqMan real-time PCR. BMDMs generated from wild-type (WT, a) or Tlr4 knockout (KO, b) mice were cultured under low-glucose (100 mg/dl, white bars) or high-glucose (450 mg/dl, black bars) conditions, and were stimulated with palmitate (0, 10, 50, and 200 μM, respectively, from the left) for 24 h. Data are mean ± SEM. n = 6. *p < 0.05. Modified from Kuwabara and others [5]
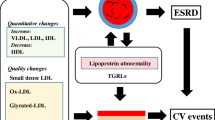
Proposed mechanism of macrophage-mediated glucolipotoxicity in diabetic nephropathy. Hyperlipidemia (or high free fatty acids) activates circulating macrophages through TLR4-mediated upregulation of MRP8, specifically under hyperglycemic conditions. These synergistic effects upon MRPã8 production in macrophages might be mediated by fetuin A and transcription factors AP-1 and CEBP/β. Macrophage activation is enhanced by a positive feedback, mediated by MRP8/TLR4 interaction in an autocrine fashion. Since glomerular intrinsic cells (such as podocytes, mesangial cells and endothelial cells) reportedly express TLR4, they can be activated through multiple pathways including (1) MRP8 from blood circulation, (2) MRP8 and inflammatory cytokines produced by glomerulus-infiltrating macrophages, and (3) hyperlipidemia. Activation of glomerular cells results in mesangial expansion and podocyte injury, further leading to glomerular sclerosis (fibrosis) and albuminuria
To understand the clinical implication of MRP8 expression in humans, we have carried out immunohistochemical analysis of MRP8 expression in renal biopsy samples from patients with DN, obesity-related glomerulopathy (ORG) and non-obese, non-diabetic controls (which are minor glomerular abnormality [MGA] and minimal change nephrotic syndrome [MCNS]). We have not been able to obtain reliable antibody against TLR4 to date. The rank orders of glomerular and tubulointerstitial MRP8 protein expression levels are DN > ORG > MCNS > MGA. Glomerular MRP8 expression is strongly correlated to the extent of proteinuria at 1 year after renal biopsy, whereas tubulointerstitial MRP8 expression is associated with worsening of renal function within a year, suggesting that renal MRP8 expression may become a new biomarker for DN (submitted).
The role of M1 and M2 macrophages in DN with glucolipotoxicity
There are several subtypes of macrophages including M1 and M2 in tissue injury and repair [72–74]. During the course of renal ischemia/reperfusion injury [75] and unilateral ureteral obstruction [76], switch from proinflammatory M1 to anti-inflammatory or profibrotic M2 subtype occurs in macrophages infiltrating the tubulointerstitium. Here, we have carried out preliminary analysis of M1 and M2 macrophages in glomeruli of STZ + HFD mice by studying gene expression levels of CD11c (or Itgax) and CD206 (or Mrc1) as markers of M1 and M2 subtypes, respectively [77, 78] (Fig. 6). In wild-type mice, treatment with STZ alone does not affect glomerular expression of CD11c and CD206 genes, and addition of HFD to STZ causes a 100 % increase in CD11c and a 30 % increase in CD206, suggesting relative predominance of M1 subtype in diabetic-hyperlipidemic conditions. Furthermore, in Tlr4 KO mice, the stimulatory effects of HFD upon STZ treatment are canceled both for CD11c and CD206 genes, and simple STZ treatment increases CD11c expression by two-fold and increases CD206 expression by three-fold, suggesting the presence of M2 predominant status. These results imply that TLR4-mediated signal is partially suppressing M2 subtype in STZ-normal diet mice and enhancing M1 subtype in STZ-HFD mice. These findings are in good agreement with previous reports indicating that treatment of macrophages with MRP8 induces M1 subtype (through TLR4 as lipopolysaccharide does) [61, 72, 76] and MRP8-expressing macrophages exhibits M1 characteristics by secretion of TNF-α and interleukin-6 [74, 79]. Formally, M1/M2 subtype analysis had to be carried out by analyzing isolated macrophages extracted from tissues.
Furthermore, in STZ + HFD animals, the levels of macrophage infiltration and extracellular matrix accumulation are proportional and progressive, suggesting that M1–M2 switching does not occur spontaneously in this model of DN. In glomeruli of STZ + HFD mice, >80 % of MRP8 signals co-localize with macrophage marker Mac2 (or Lgals3) [5], whereas collecting duct epithelial cells are the main source of MRP8 expression in unilateral ureteral obstruction [76].
In conclusion, a number of epidemiological and experimental studies have revealed that glucotoxicity and lipotoxicity cause synergistic effects upon the development and progression of DN. Macrophages have emerged as a potential contributor for mediating glucolipotoxicity through activation of MRP8/TLR4 signaling in diabetic glomeruli in our experiments. Although further studies are needed to understand regulation and potential role of MRP8/TLR4 signaling, targeting key molecules involved in this pathway may lead to novel therapeutic strategy to combat DN.
References
Tolonen N, Forsblom C, Thorn L, Waden J, Rosengard-Barlund M, Saraheimo M, Feodoroff M, Makinen VP, Gordin D, Taskinen MR, Groop PH. Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia. 2009;52:2522–30.
Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–93.
Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998–1004.
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK prospective diabetes study 74. Diabetes. 2006;55:1832–9.
Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Ogawa Y, Imamaki H, Kawanishi T, Ishii A, Koga K, Mori KP, Kato Y, Sugawara A, Nakao K. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia. 2012;55:2256–66.
Maeda S, Kobayashi MA, Araki S, Babazono T, Freedman BI, Bostrom MA, Cooke JN, Toyoda M, Umezono T, Tarnow L, Hansen T, Gaede P, Jorsal A, Ng DP, Ikeda M, Yanagimoto T, Tsunoda T, Unoki H, Kawai K, Imanishi M, Suzuki D, Shin HD, Park KS, Kashiwagi A, Iwamoto Y, Kaku K, Kawamori R, Parving HH, Bowden DW, Pedersen O, Nakamura Y. A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet. 2010;6:e1000842.
Tang SC, Leung VT, Chan LY, Wong SS, Chu DW, Leung JC, Ho YW, Lai KN, Ma L, Elbein SC, Bowden DW, Hicks PJ, Comeau ME, Langefeld CD, Freedman BI. The acetyl-coenzyme A carboxylase beta (ACACB) gene is associated with nephropathy in Chinese patients with type 2 diabetes. Nephrol Dial Transplant. 2010;25:3931–4.
Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5:2373–9.
Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–25.
Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–31.
Hou CJ, Tsai CH, Su CH, Wu YJ, Chen SJ, Chiu JJ, Shiao MS, Yeh HI. Diabetes reduces aortic endothelial gap junctions in ApoE-deficient mice: simvastatin exacerbates the reduction. J Histochem Cytochem. 2008;56:745–52.
Fledderus JO, van Oostrom O, de Kleijn DP, den Ouden K, Penders AF, Gremmels H, de Bree P, Verhaar MC. Increased amount of bone marrow-derived smooth muscle-like cells and accelerated atherosclerosis in diabetic apoE-deficient mice. Atherosclerosis. 2013;226:341–7.
Lassila M, Seah KK, Allen TJ, Thallas V, Thomas MC, Candido R, Burns WC, Forbes JM, Calkin AC, Cooper ME, Jandeleit-Dahm KA. Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: role of advanced glycation end products. J Am Soc Nephrol. 2004;15:2125–38.
Watson AM, Gray SP, Jiaze L, Soro-Paavonen A, Wong B, Cooper ME, Bierhaus A, Pickering R, Tikellis C, Tsorotes D, Thomas MC, Jandeleit-Dahm KA. Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing RAGE activation in diabetic apolipoprotein E knockout mice. Diabetes. 2012;61:2105–13.
Lopez-Parra V, Mallavia B, Lopez-Franco O, Ortiz-Munoz G, Oguiza A, Recio C, Blanco J, Nimmerjahn F, Egido J, Gomez-Guerrero C. Fcgamma receptor deficiency attenuates diabetic nephropathy. J Am Soc Nephrol. 2012;23:1518–27.
Beriault DR, Sharma S, Shi Y, Khan MI, Werstuck GH. Glucosamine-supplementation promotes endoplasmic reticulum stress, hepatic steatosis and accelerated atherogenesis in apoE−/− mice. Atherosclerosis. 2011;219:134–40.
McAlpine CS, Bowes AJ, Khan MI, Shi Y, Werstuck GH. Endoplasmic reticulum stress and glycogen synthase kinase-3beta activation in apolipoprotein E-deficient mouse models of accelerated atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:82–91.
Goldberg IJ, Hu Y, Noh HL, Wei J, Huggins LA, Rackmill MG, Hamai H, Reid BN, Blaner WS, Huang LS. Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes. Diabetes. 2008;57:1674–82.
Spencer MW, Muhlfeld AS, Segerer S, Hudkins KL, Kirk E, LeBoeuf RC, Alpers CE. Hyperglycemia and hyperlipidemia act synergistically to induce renal disease in LDL receptor-deficient BALB mice. Am J Nephrol. 2004;24:20–31.
Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, Bariéty J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–75.
Sauve M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–73.
Hazra S, Rasheed A, Bhatwadekar A, Wang X, Shaw LC, Patel M, Caballero S, Magomedova L, Solis N, Yan Y, Wang W, Thinschmidt JS, Verma A, Li Q, Levi M, Cummins CL, Grant MB. Liver x receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes. 2012;61:3270–9.
Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp Diabetes Res. 2011;2011:848307.
Zeng XY, Wang YP, Cantley J, Iseli TJ, Molero JC, Hegarty BD, Kraegen EW, Ye Y, Ye JM. Oleanolic acid reduces hyperglycemia beyond treatment period with Akt/FoxO1-induced suppression of hepatic gluconeogenesis in type-2 diabetic mice. PLoS One. 2012;7:e42115.
Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–81.
Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–60.
Suganami T, Mukoyama M, Mori K, Yokoi H, Koshikawa M, Sawai K, Hidaka S, Ebihara K, Tanaka T, Sugawara A, Kawachi H, Vinson C, Ogawa Y, Nakao K. Prevention and reversal of renal injury by leptin in a new mouse model of diabetic nephropathy. FASEB J. 2005;19:127–9.
Keren P, George J, Keren G, Harats D. Non-obese diabetic (NOD) mice exhibit an increased cellular immune response to glycated-LDL but are resistant to high fat diet induced atherosclerosis. Atherosclerosis. 2001;157:285–92.
Fox TE, Bewley MC, Unrath KA, Pedersen MM, Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, Flanagan JM, Kester M. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res. 2011;52:509–17.
Colombo C, Haluzik M, Cutson JJ, Dietz KR, Marcus-Samuels B, Vinson C, Gavrilova O, Reitman ML. Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. J Biol Chem. 2005;278:3992–9.
Breyer MD, Bottinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45.
Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–12.
Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–37.
Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991;266:10796–801.
Nakamuta M, Oka K, Krushkal J, Kobayashi K, Yamamoto M, Li WH, Chan L. Alternative mRNA splicing and differential promoter utilization determine tissue-specific expression of the apolipoprotein B mRNA-editing protein (Apobec1) gene in mice. Structure and evolution of Apobec1 and related nucleoside/nucleotide deaminases. J Biol Chem. 1995;270:13042–56.
Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45:273–83.
Brun T, Roche E, Assimacopoulos-Jeannet F, Corkey BE, Kim KH, Prentki M. Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic beta-cell nutrient signaling. Diabetes. 1996;45:190–8.
Poitout V, Robertson RP. Minireview. Secondary beta-cell failure in type 2 diabetes–a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–42.
Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–33.
El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–63.
Martinez-Garcia C, Izquierdo A, Velagapudi V, Vivas Y, Velasco I, Campbell M, Burling K, Cava F, Ros M, Oresic M, Vidal-Puig A, Medina-Gomez G. Accelerated renal disease is associated with the development of metabolic syndrome in a glucolipotoxic mouse model. Dis Model Mech. 2012;5:636–48.
Yamabe N, Noh JS, Park CH, Kang KS, Shibahara N, Tanaka T, Yokozawa T. Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. Eur J Pharmacol. 2010;648:179–87.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6.
Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61.
Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS One. 2009;4:e4954.
Lee SJ, Choi SE, Hwang YC, Jung IR, Yi SA, Jung JG, Ku JM, Jeoung K, Han SJ, Kim HJ, Kim DJ, Lee KW, Kang Y. A compound (DW1182v) protecting high glucose/palmitate-induced glucolipotoxicity to INS-1 beta cells preserves islet integrity and improves hyperglycemia in obese db/db mouse. Eur J Pharmacol. 2012;696:187–93.
Lu M, Seufert J, Habener JF. Pancreatic beta-cell-specific repression of insulin gene transcription by CCAAT/enhancer-binding protein beta. Inhibitory interactions with basic helix-loop-helix transcription factor E47. J Biol Chem. 1997;272:28349–59.
Fontes G, Semache M, Hagman DK, Tremblay C, Shah R, Rhodes CJ, Rutter J, Poitout V. Involvement of Per-Arnt-Sim kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells. Diabetes. 2009;58:2048–58.
Zador IZ, Hsieh CC, Papaconstantinou J. Renal CCAAT/enhancer-binding proteins in experimental diabetes mellitus. Nephron. 1998;79:312–6.
Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–75.
Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–55.
Endoh Y, Chung YM, Clark IA, Geczy CL, Hsu K. IL-10-dependent S100A8 gene induction in monocytes/macrophages by double-stranded RNA. J Immunol. 2009;182:2258–68.
Kuruto-Niwa R, Nakamura M, Takeishi K, Nozawa R. Transcriptional regulation by C/EBP alpha and -beta in the expression of the gene for the MRP14 myeloid calcium binding protein. Cell Struct Funct. 1998;23:109–18.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25.
Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9.
Kim JK. Fat uses a TOLL-road to connect inflammation and diabetes. Cell Metab. 2006;4:417–9.
Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–85.
Ix JH, Biggs ML, Mukamal KJ, Kizer JR, Zieman SJ, Siscovick DS, Mozzaffarian D, Jensen MK, Nelson L, Ruderman N, Djousse L. Association of fetuin-a with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation. 2012;125:2316–22.
Ou HY, Wu HT, Hung HC, Yang YC, Wu JS, Chang CJ. Endoplasmic reticulum stress induces the expression of fetuin-A to develop insulin resistance. Endocrinology. 2012;153:2974–84.
Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–2.
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9.
Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–36.
Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713–7.
Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92.
Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97.
Brown HJ, Lock HR, Wolfs TG, Buurman WA, Sacks SH, Robson MG. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J Am Soc Nephrol. 2007;18:1732–9.
Allam R, Lichtnekert J, Moll AG, Taubitz A, Vielhauer V, Anders HJ. Viral RNA and DNA trigger common antiviral responses in mesangial cells. J Am Soc Nephrol. 2009;20:1986–96.
Hagele H, Allam R, Pawar RD, Reichel CA, Krombach F, Anders HJ. Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am J Pathol. 2009;175:1896–904.
Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD, Stoelcker B, Liu G, Grone HJ, Kramer BK, Alpers CE. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–13.
Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, Meyer TN, Schwesinger CM, Akis N, Grone HJ, Anders HJ. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology. 2009;128:e206–21.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;1(122):787–95.
Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–30.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26.
Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–41.
Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, Hirata Y, Kuziel WA, Takeya M, Kanegasaki S, Kamei Y, Ogawa Y. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. 2008;19(283):35715–23.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84.
Mahnke K, Bhardwaj R, Sorg C. Heterodimers of the calcium-binding proteins MRP8 and MRP14 are expressed on the surface of human monocytes upon adherence to fibronectin and collagen. Relation to TNF-alpha, IL-6, and superoxide production. J Leukoc Biol. 1995;57:63–71.
Acknowledgments
This work was supported in part by Grant-in-Aid for Diabetic Nephropathy and Nephrosclerosis Research from the Ministry of Health, Labour and Welfare of Japan (KM), research grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (TK, KM and MM), from the Japan Foundation for Applied Enzymology (TK), from the Smoking Research Foundation (MM) and from the ONO Medical Research Foundation (TK).
Conflict of interest
The authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kuwabara, T., Mori, K., Mukoyama, M. et al. Macrophage-mediated glucolipotoxicity via myeloid-related protein 8/toll-like receptor 4 signaling in diabetic nephropathy . Clin Exp Nephrol 18, 584–592 (2014). https://doi.org/10.1007/s10157-013-0922-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-013-0922-5