Abstract
Background
Uric acid (UA) remains a risk factor of chronic kidney disease (CKD). Therefore, it is important to clarify the mechanism of UA excretion in CKD. The specific mechanisms of extrarenal excretion from the intestine are unknown. We evaluated the expression of the UA transporter in the intestinal tract—the ATP-binding cassette transporter G2 (ABCG2)—in a 5/6 nephrectomy rat model of CKD.
Methods
Male Wistar rats (6 weeks old) were randomly assigned to the 5/6 nephrectomized (Nx) group or the sham-operated control group. Urine and blood samples were collected every 4 weeks. All the rats were killed at 8 weeks to obtain liver, duodenum, jejunum, ileum, and transverse colon tissues. Uricase activity was measured in the liver. Expression of ABCG2 in intestinal mucosa was measured with real time polymerase chain reaction (PCR).
Results
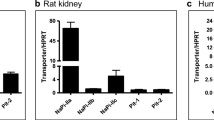
The Nx group showed significantly decreased urine UA excretion/body weight and UA clearance compared to the control group at 4 and 8 weeks after nephrectomy. In contrast, serum UA and uricase activity were not significant. The expression of ABCG2 in the ileum of the Nx group showed significantly increased upregulation, while no changes were seen in the intestines of the control group.
Conclusions
The Nx rats exhibited lower excretion of urine UA and over-expression of ABCG2 in the ileum. The fact that serum UA did not increase despite the decrease in UA excretion suggests that an excretory pathway other than the kidney, probably the intestine, may operate in a complementary role that corroborates the increase in ABCG2 expression in the ileum.




Similar content being viewed by others
References
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266.
Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–13.
Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33:1337–43.
Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7.
Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995;6:1313–7.
Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52.
Li S, Sanna S, Maschio A, et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3:e194.
Doring A, Gieger C, Mehta D, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–6.
Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42.
Anzai N, Ichida K, Jutabha P, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283:26834–8.
Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83:744–51.
Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61.
Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504.
Kamatani Y, Matsuda K, Okada Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42:210–5.
Huls M, Brown CD, Windass AS, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–5.
Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64.
Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22:7340–58.
Ichida K, Matsuo H, Takada T, et al. Decreased extrarenal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764.
Madsen S, Olgaard K, Thaysen JH. The effect of 1-alpha-hydroxycholecalciferol on the renal handling of phosphate in parathyroidectomized man. Acta Med Scand. 1977;202:23–6.
Yamamoto M, Kawanobe Y, Takahashi H, et al. Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest. 1984;74:507–13.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21.
Edwards NL. The role of hyperuricemia in vascular disorders. Curr Opin Rheumatol. 2009;21:132–7.
Boss GR, Seegmiller JE. Hyperuricemia and gout. Classification, complications and management. N Engl J Med. 1979;300:1459–68.
Sorensen LB. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 1965;8:694–706.
Sorensen LB, Levinson DJ. Origin and extrarenal elimination of uric acid in man. Nephron. 1975;14:7–20.
Woodward OM, Kottgen A, Coresh J, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA. 2009;106:10338–42.
Matsuo H, Takada T, Ichida K, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5ra11.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan (to SU) and was also supported in part by the Torii Scientific Award for Gout Research (to SU).
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yano, H., Tamura, Y., Kobayashi, K. et al. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin Exp Nephrol 18, 50–55 (2014). https://doi.org/10.1007/s10157-013-0806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-013-0806-8