Abstract
Background
It has been reported matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMPs), play important roles in the decomposition of the extracellular matrices of the glomerulus during the pathological processes in various glomerular diseases. Although the activity of these enzymes in cases of experimental glomerulonephritis has been described, the expression sites in the glomeruli of human renal diseases have been identified in only a few articles and remain controversial.
Methods
The expression of the gelatinase group of MMPs (MMP-2 and MMP-9) and their inhibitors (TIMP-2 and TIMP-1) were evaluated in 19 renal biopsies of several types of glomerular diseases by immunofluorescence (IF) labeling. In addition, several samples of immunoglobulin A nephropathy (IgAN) were also investigated by in situ hybridization (ISH) and immunoelectron microscopy (IEM).
Results
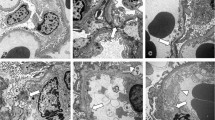
The expression of MMP-2 was observed in all the cases examined by IF and ISH. TIMP-2 expression varied from negative to positive among 11 cases of IgAN, but was negative in the cases with lupus nephritis (LN) (n = 3), membranoproliferative glomerulonephritis (MPGN) (n = 2), and post-streptococcal glomerulonephritis (n = 1). However, it was weakly positive in the cases of diabetic nephropathy (DMN) (n = 2). MMP-2 was mainly observed along glomerular capillary loops (GCLs) and Bowman’s capsules, whereas TIMP-2 was found in the mesangial area. The expression of MMP-9 in cases of IgAN varied, and was local, not diffuse, if it was present. MMP-9 expression in cases of LN, MPGN, and DMN was diffuse, but the intensity of staining varied. MMP-9 was primarily expressed in the mesangium. TIMP-1 expression was negative in all cases except for those with IgAN. The localization of MMP-2 in patients with IgAN, which was investigated by IEM, was revealed to be mainly on the endothelial cell membranes of GCLs, podocyte membranes, the parietal cell membranes of Bowman’s capsules, and some on the membranes of mesangial cells.
Conclusion
The study results suggest that the expression levels and patterns of MMPs and TIMPs are generally similar in several types of glomerular diseases, even though each case has a somewhat different distribution and intensity of expression. When these enzymes were present, their main sites were as follows: MMP-2 was found along glomerular basement membrane, TIMP-2 was located in the acellular mesangial area, MMP-9 was seen in the mesangium, and TIMP-1 was hardly detected. MMP-2 expression is clearly demonstrated to exist at the above-described sites by IEM in patients with IgAN.




Similar content being viewed by others
References
Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54.
Marti HP. The role of matrix metalloproteinases in the activation of mesangial cells. Transpl Immunol. 2002;9:97–100.
Schnaper HW. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr Nephrol. 1995;9:104–11.
Illman SA, Lohi J, Keski-Oja J. Epilysin (MMP-28)-structure, expression and potential functions. Exp Dermatol. 2008;17:897–907.
Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–81.
Conway JG, Wakefield JA, Brown RH, Marron BE, Sekut L, Stimpson SA, et al. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med. 1995;182:449–57.
O-Charoenrat P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–68.
Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71.
Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83.
Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272:29975–83.
Lovett DH, Johnson RJ, Marti HP, Martin J, Davies M, Couser WG. Structural characterization of the mesangial cell type IV collagenase and enhanced expression in a model of immune complex-mediated glomerulonephritis. Am J Pathol. 1992;141:85–98.
McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH. Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J Clin Invest. 1996;97:1094–101.
Steinmann-Niggli K, Ziswiler R, Küng M, Marti HP. Inhibition of matrix metalloproteinases attenuates anti-Thy1.1 nephritis. J Am Soc Nephrol. 1998;9:397–407.
Schaefer L, Han X, August C, Matzkies F, Lorenz T, Schaefer RM. Differential regulation of glomerular gelatinase B (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in obese Zucker rats. Diabetologia. 1997;40:1035–43.
Martin J, Knowlden J, Davies M, Williams JD. Identification and independent regulation of human mesangial cell metalloproteinases. Kidney Int. 1994;46:877–85.
Steinmann-Niggli K, Lukes M, Marti HP. Rat mesangial cells and matrix metalloproteinase inhibitor: inhibition of 72-kD type IV collagenase (MMP-2) and of cell proliferation. J Am Soc Nephrol. 1997;8:395–405.
Martin J, Steadman R, Knowlden J, Williams J, Davies M. Differential regulation of matrix metalloproteinases and their inhibitors in human glomerular epithelial cells in vitro. J Am Soc Nephrol. 1998;9:1629–37.
Lenz O, Striker LJ, Jacot TA, Elliot SJ, Killen PD, Striker GE. Glomerular endothelial cells synthesize collagens but little gelatinase A and B. J Am Soc Nephrol. 1998;9:2040–7.
Wu K, Setty S, Mauer SM, Killen P, Nagase H, Michael AF, et al. Altered kidney matrix gene expression in early stages of experimental diabetes. Acta Anat (Basel). 1997;158:155–65.
Shiozawa S. Participation of macrophages in glomerular sclerosis through the expression and activation of matrix metalloproteinases. Pathol Int. 2000;50:441–57.
Miyazaki M, Koji T, Furusu A, Abe K, Ozono Y, Harada T, et al. In situ hybridization studies of stromelysin and tissue inhibitor of metalloproteinase 1 in IgA nephropathy. Nephrology. 1995;1:119–27.
Sakai H, Jinde K, Suzuki D, Yagame M, Nomoto Y. Localization of glycated proteins in the glomeruli of patients with diabetic nephropathy. Nephrol Dial Transplant. 1996;11(Suppl 5):66–71.
Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, et al. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 1997;52:111–9.
Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, et al. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia. 1997;40:1449–54.
Suzuki D, Yagame M, Kim Y, Sakai H, Mauer M. Renal in situ hybridization studies of extracellular matrix related molecules in type 1 diabetes mellitus. Nephron. 2002;92:564–72.
Urushihara M, Kagami S, Kuhara T, Tamaki T, Kuroda Y. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol Dial Transplant. 2002;17:1189–96.
Wang J, Chen X, Shi S, Zhang Y, Tian Y. Expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in IgA nephropathy. Chin J Intern Med. 2002;41:75–8 (in Chinese, Abstract in English).
Endo T, Nakabayashi K, Sekiuchi M, Kuroda T, Soejima A, Yamada A. Matrix metalloproteinase-2, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in the peripheral blood of patients with various glomerular diseases and their implication in pathogenetic lesions: study based on an enzyme-linked assay and immunohistochemical staining. Clin Exp Nephrol. 2006;10:253–61.
Jalalah SM, Furness PN, Barker G, Thomas M, Hall LL, Bicknell GR, et al. Inactive matrix metalloproteinase 2 is a normal constituent of human glomerular basement membrane. An immuno-electron microscopic study. J Pathol. 2000;191:61–6.
Kudo A, Fukushima H, Kawakami H, Matsuda M, Goya T, Hirano H. Use of serial semithin frozen sections to evaluate the co-localization of estrogen receptors and progesterone receptors in cells of breast cancer tissues. J Histochem Cytochem. 1996;44:615–20.
Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–66.
Tomino Y, Sakai H. Special Study Group (IgA Nephropathy) on Progressive Glomerular Disease. Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol. 2003;7:93–7.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–45.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56.
Kuroda T, Yoshida Y, Kamiie J, Kovalenko P, Nameta M, Fujinaka H, et al. Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis in WKY rats. Clin Exp Nephrol. 2004;8:206–15.
Waku M, Nakabayashi K, Karube M, Nagasawa T. Serum level of human tissue inhibitor metalloproteinase-1 in various glomerulonephritides. J Kyorin Med Soc. 1997;28:441–7 (in Japanese, Abstract in English).
Acknowledgments
We are grateful to Mr. M. Fukuda, Ms. S Matsubara (Laboratory for Electron Microscopy), Ms. T. Miura (Department of Anatomy), and Ms. K. Sano (First Department of Internal Medicine) for their excellent technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sekiuchi, M., Kudo, A., Nakabayashi, K. et al. Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix metalloproteinases 2 and 1 in the glomeruli of human glomerular diseases: the results of studies using immunofluorescence, in situ hybridization, and immunoelectron microscopy. Clin Exp Nephrol 16, 863–874 (2012). https://doi.org/10.1007/s10157-012-0633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0633-3