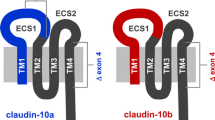
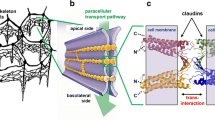
Abstract
Tight junctions (TJs) are the most apical component of junctional complexes and regulate the movement of electrolytes and solutes by the paracellular pathway across epithelia. The defining ultrastructural features of TJs are strands of transmembrane protein particles that adhere to similar strands on adjacent cells. These strands are mainly composed of linearly polymerized integral membrane proteins called claudins. Claudins comprise a multigene family consisting of more than 20 members in mammals. Recent work has shown that claudins form barriers, determined by the paracellular electrical resistance and charge selectivity, and pores in the TJ strands. The paracellular pathways in renal tubular epithelia such as the proximal tubule, which reabsorbs the largest fraction of filtered NaCl and water, are important routes for the transport of electrolytes and water. Their transport characteristics vary among different nephron segments. Multiple claudins are expressed at TJs of individual nephron segments in a nephron segment-specific manner. Among them, claudin-2 is highly expressed at TJs of proximal tubules, which are leaky epithelia. Overexpression and knockdown of claudin-2 in epithelial cell lines, and knockout of the claudin-2 gene in mice, have demonstrated that claudin-2 forms high-conductance cation-selective pores in the proximal tubule. Here, we review the renal physiology of paracellular transport and the physiological roles of claudins in kidney function, especially claudin-2 and proximal tubule paracellular NaCl transport.


Similar content being viewed by others
References
Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29.
Tsukita S, Furuse M, Ito H. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93.
Angelow S, Ahlstrom R, Yu ASL. Biology of claudins. Am J Physiol. 2008;295:F867–76.
Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412.
Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241:G275–88.
Farquhar MG, Palade GE. Cell junctions in amphibian skin. J Cell Biol. 1965;26:263–91.
Staehelin LA. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283.
Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol. 1973;58:390–400.
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88.
Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–49.
Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112:1879–88.
Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, et al. Occludin deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408.
Saitou M, Furuse M, Sasaki H, Schulzke JK, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–442.
Furuse M, Fujita K, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50.
Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401.
Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204.
Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol. 2002;283:C142–7.
Colegio OR, Van Itallie CM, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol. 2003;284:C1346–54.
Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol. 2003;285:F1078–84.
Van Itallie CM, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–27.
Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305.
Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl− permeability. Biochem Biophys Res Commun. 2007;357:87–91.
Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–93.
Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–6.
Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, et al. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–22.
Imai M. Function of the thin ascending limb of Henle of rats and hamsters in vitro. Am J Physiol. 1977;1:F201–9.
Sansom SC, Weinman EJ, O’Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984;247:F291–302.
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–86.
Enck AH, Berger UV, Yu ASL. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol. 2001;281:F966–74.
Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol. 2006;291:F1288–99.
Li WY, Huey CL, Yu ASL. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol. 2004;286:F1063–71.
Angelow S, El-Husseini R, Kanzawa SA, Yu ASL. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol. 2007;293:F166–77.
Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–57.
Rector FC Jr. Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F4611–711.
Liu FY, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol. 1984;247:F816–21.
Berry CA, Rector FC Jr. Relative sodium-to-chloride permeability in the proximal convoluted tubule. Am J Physiol. 1978;4:F592–604.
Schafer JA, Troutman SL, Andreoli TE. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol. 1974;64:582–607.
Jacobson HR, Kokko JP. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976;57:818–25.
Kawamura S, Imai M, Seldin DW, Kokko JP. Characteristics of salt and water transport in superficial and juxtamedullary straight segments of proximal tubules. J Clin Invest. 1975;55:1269–77.
Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA. 2010;107:8011–6.
Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into MDCK I cells. J Cell Biol. 2001;153:263–72.
Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76.
Yu ASL, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interacting site. J Gen Physiol. 2009;133:111–27.
Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–23.
Acknowledgments
We would like to express our sincere gratitude and deep appreciation for the support and encouragement from the late Prof. Shoichiro Tsukita, without which this research would have been impossible. This work was funded by Grants-in-Aid from the Ministry of Education, Science, Culture, Sports, Science, and Technology of Japan and by a grant from the Salt Science Foundation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Muto, S., Furuse, M. & Kusano, E. Claudins and renal salt transport. Clin Exp Nephrol 16, 61–67 (2012). https://doi.org/10.1007/s10157-011-0491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0491-4