Abstract
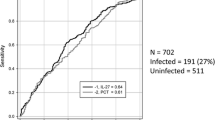
The clinical usefulness of presepsin for discriminating between bacterial and nonbacterial infections (including systemic inflammatory response syndrome) was studied and compared with procalcitonin (PCT) and interleukin-6 (IL-6) in a multicenter prospective study. Suspected sepsis patients (n = 207) were enrolled into the study. Presepsin levels in patients with systemic bacterial infection and localized bacterial infection were significantly higher than in those with nonbacterial infections. In addition, presepsin, PCT, and IL-6 levels in patients with bacterial infectious disease were significantly higher than in those with nonbacterial infectious disease (P < 0.0001, P < 0.0001, and P < 0.0001, respectively). The area under the receiver operating characteristic curve was 0.908 for presepsin, 0.905 for PCT, and 0.825 for IL-6 in patients with bacterial infectious disease and those with nonbacterial infectious disease. The cutoff value of presepsin for discrimination of bacterial and nonbacterial infectious diseases was determined to be 600 pg/ml, of which the clinical sensitivity and specificity were 87.8 % and 81.4 %, respectively. Presepsin levels did not differ significantly between patients with gram-positive and gram-negative bacterial infections. The sensitivity of blood culture was 35.4 %; that for presepsin was 91.9 %. Also there were no significant differences in presepsin levels between the blood culture-positive and -negative groups. Consequently, presepsin is useful for the diagnosis of sepsis, and it is superior to conventional markers and blood culture.



Similar content being viewed by others
References
Meisner M. Pathobiochemistry and clinical use of procalcitonin. 2002;323(1-2):17–29.
Aikawa N, Fujishima S, Endo S, Sekine I, Kogawa K, Yamamoto Y, et al. Multicenter prospective study of procalcitonin as an indicator of sepsis. J Infect Chemother. 2005;11:152–9.
Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244–9.
Endo S, Sato N, Suzuki Y, Kojika M, Takahashi G, Yamada Y, et al. Significance of measuring procalcitonin values for diagnosis of sepsis. J Jpn Soc Surg Infect 2007;4(3):112–120 (in Japanese)
Furusako S, Shirakawa K. Methods for detecting human low molecular weight CD14. United States patent 2008; US7465547 B2.
Furusako S, Shirakawa K, Hirose J. Soluble CD14 antigen. United States patent 2009; US7608684 B2.
Naitoh K, Shirakawa K, Hirose J, Nakamura M, Takeuchi T, Hosaka Y, et al. The new sepsis marker, sCD14-ST (PRESEPSIN): induction mechanism in the rabbit sepsis models. SEPSIS 2010, Poster P-19.
Nakamura M, Takeuchi T, Naito K, Shirakawa K, Hosaka Y, Yamasaki F, et al. Early elevation of plasma soluble CD14 subtype, a novel biomarker for sepsis, in a rabbit cecal ligation and puncture model. Crit Care. 2008;12(suppl 2):P194. doi:10.1186/cc6415.
Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. 2005;11(5):234–8.
Kojika M, Takahashi G, Matsumoto N, Kikkawa T, Hoshikawa K, Shioya N, et al. Serum levels of soluble CD14 subtype reflect the APACHE II and SOFA Scores. Med Postgrad. 2010;48(1):46–50.
Takahashi G, Suzuki Y, Kojika M, Matsumoto N, Shozushima T, Makabe H, et al. Evaluation of responses to IVIG therapy in patients with severe sepsis and septic shock by soluble CD14 subtype monitoring. Med Postgrad. 2010;48(1):19–24.
Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17(6):764–9.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
Kurihara T, Yanagida A, Yokoi H, Koyata A, Matsuya T, Ogawa J, et al. Evaluation of cardiac assays on a benchtop chemiluminescent enzyme immunoassay analyzer, PATHFAST. Anal Biochem. 2008;375(1):144–6.
Okamura Y, Yokoi H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta. 2011;412(23–24):2157–61.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Endo, S., Suzuki, Y., Takahashi, G. et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother 18, 891–897 (2012). https://doi.org/10.1007/s10156-012-0435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0435-2