Abstract
Larvae of many marine decapod crustaceans are released in unpredictable habitats with strong salinity fluctuations during the breeding season. In an experimental laboratory study, we investigated the influence of seven different salinities (0, 5, 10, 15, 20, 25 and 30) on the survival and development time of fiddler crab zoea larvae, Uca vocator, from northern Brazilian mangroves. The species reproduces during the rainy season when estuarine salinity strongly fluctuates and often reaches values below 10 and even 5. Salinity significantly affected the survival rate and development period from hatching to megalopa, while the number of zoeal stages remained constant. In salinities 0 and 5, no larvae reached the second zoeal stage, but they managed to survive for up to 3 (average of 2.3 days) and 7 days (average of 5.1 days), respectively. From salinity 10 onwards, the larvae developed to the megalopal stage. However, the survival rate was significantly lower (5–15%) and development took more time (average of 13.5 days) in salinity 10 than in the remaining salinities (15–30). In the latter, survival ranged from 80–95% and development took 10–11 days. Given the 100% larval mortality in extremely low salinities and their increased survival in intermediate and higher salinities, we conclude that U. vocator has a larval ‘export’ strategy with its larvae developing in offshore waters where salinity conditions are more stable and higher than in mangrove estuaries. Thus, by means of ontogenetic migration, osmotic stress and resulting mortality in estuarine waters can be avoided.
Similar content being viewed by others
Introduction
Most decapod crab species have a complex biphasic life cycle including meroplanktonic and benthonic phases. The larval development occurs during a time interval of a few days to several weeks in the plankton before transition to the benthic environment (Anger 2001, 2006). During ontogenesis, the larvae are frequently exposed to variations in temperature and salinity, as well as in food availability and predation pressure (Morgan 1995; Anger 2001, 2003, 2006). Among these environmental factors, salinity is considered a key ecological parameter that has a high variability in estuaries and marine coastal ecosystems (Anger 2003). This parameter is of extreme importance in the life of decapod crustaceans due to its influence on several aspects of larval biology, such as, survival, development, morphology, the moulting cycle, growth, feeding, metabolism, energy partitioning, and behaviour (for review see Anger 2001, 2003). Salinity may also affect the biochemical composition and embryogenesis of decapod larvae causing variability in the early larval biomass, survival and growth (Giménez and Anger 2001; Torres et al. 2007, 2008).
Although many juvenile and adult estuarine crabs are strong hyper-/hypo-osmoregulators and can tolerate a wide range of salinity in the environment, their larvae may be more sensitive to the variations in the salinity levels that occur within the estuarine waters (Charmantier 1998; Anger and Charmantier 2000; Giménez and Anger 2001). The ability of the larvae to tolerate different salinities can be used as an indicator of the type of strategy adopted by many estuarine species, as for instance, larval ‘retention’ or larval ‘export’ (Strathmann 1982; Anger 2001; Anger et al. 2008). While estuarine waters are typically less saline and often fluctuate strongly in salinity, offshore waters are generally more saline and stable. Hence, larvae of species with ‘retention’ strategy need to be more tolerant towards low and variable salinity conditions than those with ‘export’ strategy developing offshore.
Here, we study in the laboratory whether salinity influences the larval development of the neotropical fiddler crab Uca vocator (Herbst 1804) inhabiting northern Brazilian mangroves. Specifically, we investigate whether the larvae are capable to successfully develop in salinities typical for estuarine waters in the study area or whether they need more marine conditions that would indicate the larval export strategy. To our knowledge, studies addressing the effects of salinity on the ontogenesis of fiddler crab species are still limited, e.g. U. minax, U. pugnax, U. subcylindrica and U. tangeri (O’Connor and Epifanio 1985; Rabalais and Cameron 1985; Epifanio et al. 1988; Spivak and Cuesta 2009). U. vocator is an abundant semi-terrestrial crab species distributed along the Atlantic coast of the subtropical and tropical Americas (Melo 1996). In northern Brazil, the crabs live in the high- to mid-intertidal zones of mangroves in both up- and downstream areas (Melo 1996; Koch and Wolff 2002; Koch et al. 2005; Diele et al. 2010). Megalopae and juveniles are found in the same habitat as the adults. The crabs are frequently preyed upon by fishes (Giarrizzo and Saint-Paul 2008), thus being important for benthic–pelagic coupling. The larval development of this species was described by Rieger (1999) under laboratory conditions. The author observed 4, 5 or 6 zoeal and one megalopal stage when larvae were reared in salinity 34. It is not known whether this variability in the number of larval stages occurs also in other salinity regimes or whether it results from intrinsic or maternal factors.
In the mangroves of the Caeté river estuary (northern Brazil), U. vocator reproduces during the rainy season with pronounced peaks of ovigerous females occurring from March to June (Koch et al. 2005). In the study area, mean annual rainfall is 2500–3000 mm (INMET 1992; MADAM, unpublished data) and occurs mostly during the wet season (January to June) (Diele and Simith 2006). Hence, salinity is often low during the wet season and varies between 0 and 39 (Diele and Simith 2006). In addition, significant input of ‘black freshwater’ originating from the Amazon River further reduces the salinity in near-coastal waters (Simith DJB, personal observation). As a consequence of these extreme salinity fluctuations, the larvae of estuarine crabs experience osmotic stress during ontogenesis. Larval ‘export’ with subsequent development in offshore waters may therefore be the typical reproductive strategy in this environment. This assumption was already confirmed for the northern Brazilian co-occurring mangrove crab Ucides cordatus (Diele 2000; Diele and Simith 2006; Simith and Diele 2008).
In the present study, we experimentally investigate the effects of seven different salinities on (i) the survival and development period from hatching to megalopa, (ii) survival and duration of larvae in each zoeal stage, and (iii) the total number of larval stages of U. vocator. Based on the known salinity variations in the estuary of our northern Brazilian study site, the observed pattern of larval salinity tolerance of subsequent zoeal stages is used to predict the reproductive strategy of U. vocator in this environment.
Materials and methods
Seawater for larval cultivation
Seawater for larval rearing (salinity 35) was collected 16–35 km off Bragança peninsula (northern Brazil). The seawater was filtered (Eheim and Diatom Filter: 1 μm), sterilised with ultraviolet filter (Gehaka) and stored in tanks (500 L) with constant aeration in the laboratory. Sodium hypochlorite (2.5%) was added (0.5 ml/l seawater) for additional sterilisation. Different salinities for larval cultivation in the respective experimental treatments (see below) were obtained by diluting the filtered seawater with appropriate amounts of deionised tap water. Salinity was then measured with a WTW-LF 197 probe and with a hand refractometer (Atago). As the diluted water (salinities 0, 5 and 10) became slightly acidic (≤5), its pH was adjusted to values ≥7 using biological filters built with sterile substrata (e.g. shells). pH values were measured with a pH-meter probe (pH-710, Instrutherm). Prior to the cultivations, both freshwater and seawater with different salinities (5–30) were biologically filtered for several days to weeks under constant aeration to keep ammonium and nitrite levels low using the filters above.
Larval obtainment, salinity treatments and rearing conditions
Larvae of U. vocator were obtained from an ovigerous female (carapace width 2 cm) collected 1 day before hatching, at new moon in April 2007, in the mangroves of the Caeté river estuary (State of Pará, northern Brazil). After capture, the specimen was transported in an aquarium filled with mud substratum and estuarine water to the Laboratory of Aquiculture at the Campus of the Federal University of Pará (located in the city of Bragança). The crab was washed and placed in a glass aquarium with 5 L of moderately aerated filtered seawater. The female was kept in salinity 20 (same salinity as encountered in the field), at 26°C and pH 8.0 until larval release.
U. vocator larvae were cultivated in seven different salinity treatments (Sal.): (Sal. 0 = treated deionised tap water, Sal. 5, 10, 15, 20, 25 and 30). Immediately after hatching, the larvae were placed into 500-ml glass beakers where they were slowly acclimated in steps of 5 salinity units up or down (2 h per interval) from the hatching salinity (Sal. 20) to the final desired experimental salinities. An acclimation time of 1–2 h was shown to be sufficient for decapod larvae to reach a constant haemolymph concentration (see Charmantier and Charmantier-Daures 1991; Charmantier et al. 1998). After acclimation, the larvae were transferred to 250-ml plastic vials, using wide-bore pipettes, for cultivations in the respective experimental salinities. Each salinity treatment was carried out with three replicate vials containing an initial n = 20 zoea larvae each. In addition to partial water changes (see below), the cultivation water of the vials was completely replaced every 3 and in rare occasions every 4 days. After each water renewal, food was immediately added (see below). Salinity was measured daily, but did not change much between water changes, due to the high humidity and consequently low evaporation rate in the laboratory.
The larvae were cultivated to megalopal stage without aeration, at 29.5 ± 1.1°C, pH 7.9 ± 0.1 and with a photoperiod regime of 12:12 h/light:dark cycle. In the cultivations from Sal 5–30, the newly hatched zoea larvae were fed daily with microalgae Thalassiosira sp. (approximately 100 cells·ml−1) and rotifers Brachionus plicatilis (at a density of 140 ind·ml−1; enriched with microalgae Dunaliella salina). From the third zoeal stage onwards, the larvae were additionally fed with freshly hatched brine-shrimp Artemia sp. nauplii (at a density of approximately 10 nauplii·ml−1). The food items were sieved (100 μm) and rinsed with water of the same salinity as in the respective treatments before adding them to the cultivation vials. Thereby, undesired changes in the experimental salinities were avoided. In Sal. 0, larvae were exclusively fed with rotifers (after enriching by feeding with microalgae D. salina and Thalassiosira sp.). Rotifers were sieved in a 45-μm mesh size and rinsed with treated deionised tap water. They were then added to the cultivation vials. As the rotifers only remained active for approximately 12–24 h in freshwater, the frequency of feeding was increased in this treatment.
Larvae were monitored every 12 h for mortality and also microscopically checked for moults to subsequent zoeal stages based on their morphology. This was achieved by carefully collecting each larva with a wide-bore pipette and putting it into a drop of seawater on a glass slide under a compound microscope (Zeiss). After morphological inspection, each zoea larva was placed from the slide into a new plastic vial containing half of the former cultivation water. When all larvae per replicate vial had been checked and transferred to the new vial, the latter was filled with new water of the respective salinity treatment. Based on the above morphological staging of the surviving specimens, the duration of each zoeal stage was determined for each replicate vial and treatment. The different zoeal stages were identified following descriptions given by Rieger (1999). The occurrence of megalopae in the culture could be observed without microscopic inspection due to their large size and distinct morphology. Due to other space requirements in the laboratory, the experiment had to be stopped once the larvae had reached the megalopal stage. Therefore, no data concerning survival and development duration of U. vocator megalopae were obtained. Hence, the experiment was conducted until the last zoea larvae in each salinity treatment had either died or moulted to the megalopal stage.
Statistical analyses
In order to verify the possible effects of different salinities on the larval development of U. vocator, the data were first analysed comparing the total survival rates (%) and larval development duration (in days) from hatching to megalopal stage among all treatments. Thereafter, comparisons were done in order to test for differences in the moulting rates and duration of each zoeal stage among the different salinity treatments. Per cent survival in each zoeal stage was related to the number of survivors from former stages.
All statistical analysis followed standard techniques (Sokal and Rohlf 1995). The survival data were analysed by contingency tables (R × C) followed by Chi-square tests. For the analysis of development duration, the non-parametric Kruskal–Wallis’s H test was employed after checking for normality and equality of variances by means of the Kolmogorov–Smirnov’s and Levene’s tests, respectively. Multiple a posteriori comparisons were performed using the Dunn’s test for data sets with different size or Mann–Whitney’s U test in order to identify pair-wise differences among salinity treatments. Differences were considered significant when P < 0.05. Data analyses were only performed among treatments when the requirements for data (or sample) size of each statistical test were obtained at the end of the experiment (e.g. a minimum number of 5 for expected frequencies in contingency tables).
Results
Larval survival
In our experiment, the survival rate of U. vocator larvae from hatching to megalopal stage differed significantly (P < 0.05) among the seven salinity treatments (Fig. 1). All larvae died when reared in extremely low salinities (Sal. 0 and 5) (Fig. 1). The zoea larvae only completed development until megalopa when reared in salinity 10 onwards (Fig. 1). However, there were higher total survival rates from hatching to megalopa in intermediate and higher salinities (Sal. ≥15 with averages ranging from 80–95%), while the survival was significantly lower (P < 0.05) in Sal. 10 (ranging from 5–15%) (Fig. 1).
Survival of Uca vocator larvae (%, average ± standard deviation) from hatching to megalopa and for each zoeal stage (ZI–ZV) reared in seven different salinity treatments. % values of the ZI stage refer to initial n = 60 larvae equally distributed in three replicate vials per treatment, while for ZII–ZV stages values are related to initial n of each surviving zoea that moulted to the subsequent larval stage. Equal letters above bars indicate statistically homogeneous groups (P > 0.05)
The U. vocator larvae developed through five zoeal stages (ZI–ZV) to megalopa, irrespective of salinity conditions (Sal. ≥10). All newly hatched zoea larvae did not survive in Sal. 0 and 5; however, they moulted to the second zoeal stage (ZII) in all the other salinities (Fig. 1). When reared in Sal. 10, 35–100% of the ZI larvae moulted to the ZII stage, which was significantly less (P < 0.05) than in the remaining salinities, where all ZI larvae (100%) survived (Fig. 1). The pattern found for ZII, ZIII and ZV larval stages was similar to the ZI stage, presenting reduced survival rates in Sal. 10 and elevated survival in intermediate and higher salinities (Fig. 1). By contrast, the survival rates of the larvae in ZIV stage did not differ between Sal. 15, 20, 25 and 30 (averages ranged from 92–100%) (Fig. 1).
Larval development duration
Similar to survival rate, the development duration of U. vocator larvae from hatching to megalopal stage was also significantly distinct (H = 57.01; df = 3; P < 0.001) among the salinity treatments (Fig. 2). The development duration was significantly shorter (P < 0.05) in intermediate and high salinities (Sal. ≥15, averages between 10 and 11 days) and longer when specimens were reared in a lower salinity condition (Sal. 10, average of 13.5 days) (Fig. 2).
Development duration of Uca vocator larvae (in days, average ± standard deviation) from hatching to megalopa and for each zoeal stage (ZI–ZV) reared in seven different salinity treatments. Values without bar specifically refer to the time in which larvae survived until dying without moulting to the subsequent zoeal stage. Numbers inside bars refer to the number of specimens in each zoeal stage. Different letters above bars indicate significant differences (P < 0.05) after pair-wise comparisons
Although all ZI larvae eventually died in Sal. 0 and 5 without moulting to ZII stage, they still lived for an average of 2.3 and 5.1 days, respectively. In Sal. 10, the average duration of the ZI stage was 3.5 days, while it was significantly shorter (P < 0.05) in the higher salinities (≥15) (Fig. 2). The ZII and ZIII larvae also developed significantly faster (P < 0.05) in salinities from 15–30, while they took a longer time in Sal. 10 (Fig. 2). Faster development in Sal. 15–30 and slower development in Sal. 10 (average of 4.4 days) were also exhibited by the ZIV stage (Fig. 2). However, the development period in Sal. 30 did not differ significantly (P > 0.05) from the other salinity treatments (Fig. 2). Unlike the previous zoeal stages, the development of the ZV stage did not differ significantly (H = 4.72; df = 3; P > 0.05) among treatments presenting average values of around 2 and 3 days (Fig. 2).
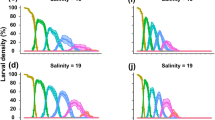
Total larval development from hatching to megalopa took 9 (Sal. 25) to 15 days (Sal. 10). By day 10 and 11 > 50% of the larvae had reached the megalopal stage in all salinities ≥15 (Fig. 3). Despite total mortality in Sal. 0 and 5, the ZI stage still lived some days in these extremely low salinities (Fig. 3). In Sal. 0, the ZI larvae suffered an abrupt mortality at day 3 of cultivation and all larvae had already died 1 day later (Fig. 3). In Sal. 5, the first event of mortality occurred on day 2 onwards and some ZI larvae remained alive for up to 7 days before dying without moulting to the subsequent zoeal stage (Fig. 3). The development period and the survival curves of each zoeal stage of Uca vocator through successive days of cultivation in the respective salinity treatments are shown in Fig. 3.
Survival (%) and development time (days) of Uca vocator larvae through successive zoeal stages (ZI–ZV) during cultivation in seven different salinity treatments. % values of subsequent zoeal stages refer to the initial n = 60 larvae per treatment (data of the three replicate vials per treatment were pooled). Results are shown until day 15 when the last ZV larva had moulted to megalopal stage
Discussion
Salinity has been considered as an important environmental factor in the larval biology and ecology of many decapod crustaceans due to its variability in estuaries and other marine coastal ecosystems (Anger 2001, 2003). During the successive phases of the early development of its life cycle, U. vocator may be subject to different salinities in the planktonic environment during the breeding season in northern Brazil. In the present study, we demonstrate that larval survival and development time until the megalopal stage of U. vocator are significantly affected by different salinities. Salinity also affects the larval survival in many other crab species (e.g. Uca minax, U. pugnax and U. tangeri: O’Connor and Epifanio 1985; Epifanio et al. 1988; Spivak and Cuesta 2009; Sesarma angustipes: Anger et al. 1990; Eriocheir sinensis: Anger 1991; Armases rubripes: Luppi et al. 2003; Ucides cordatus: Diele and Simith 2006; Neohelice (=Chasmagnathus) granulata: Anger et al. 2008) and also the larval development duration (e.g. Uca minax, U. pugnax, and U. tangeri: O’Connor and Epifanio 1985; Epifanio et al. 1988; Spivak and Cuesta 2009; Armases miersii: Anger 1996; A. rubripes: Luppi et al. 2003). In some cases, the prolonged development period comprises an additional larval stage when larvae are reared in low salinities (e.g. E. sinensis: Anger 1991; N. granulata: Giménez 2003; Giménez and Anger 2003). However, in our experiment, salinity did not influence the number of larval stages of U. vocator. The larvae always developed via five zoeal stages to megalopa, irrespective of salinity (≥10). Nevertheless, as demonstrated by Rieger (1999), the number of zoeal stages in the larval development of U. vocator can also be flexible. In his study, the larvae (from a southwestern Brazilian crab population) were cultivated individually in a constant salinity of 34 (25 ± 1°C) and developed via 4, 5 or 6 zoeal stages. In the absence of competition for space and food, as is the case when larvae are reared individually, as well as under optimal nutritional conditions, an abbreviated development with reduced number of larval stages may occur. Some of Rieger’s individually reared larvae, however, developed via one extra stage compared to our mass-cultivated specimens, despite similar food quality and quantity. Thus, we can exclude the hypothesis of the influence of competition or of different diets. The observed flexibility in the developmental pathway of U. vocator may instead result from intrinsic factors such as genetic variability between larvae from different broods (from the same or from geographically different populations) or between siblings and/or it may result from maternal differences (e.g. health, fitness) rather than from extrinsic determinants, such as salinity. In the present study, no information on intraspecific variability could be obtained as all larvae were siblings. Data on intraspecific variability in response to environmental factors such as salinity or temperature require that larvae from different broods are studied, which will be considered in future experiments.
The effect of salinity on the development of brachyuran crab larvae can indicate the larval ‘export’ or larval ‘retention’ strategy in estuarine species (Strathmann 1982). In the present study, the U. vocator larvae from the northern Brazilian Caeté estuary showed higher survival rates and faster development from hatching to megalopal stage in intermediate and high salinities (≥15), while all larvae died when reared in extremely reduced salinities (0 and 5) and only 10% survived in salinity 10 after a prolonged period of development. In most mangrove creeks of the Caeté estuary, including the outermost creeks, salinity regularly falls below 10 during the peak wet season months and may even reach values below 5 (see Figs. 1, 2, 3 in Diele and Simith 2006, data source 2001–2005). In this estuary, the reproductive peak of U. vocator coincides exactly with the period of heavy tropical rainfall (Koch et al. 2005) and abrupt salinity fluctuations (Diele and Simith 2006). Our results strongly suggest that U. vocator larvae cannot survive in such an environment due to osmotic stress and low salinities caused by heavy rainfalls. So given the stable adult populations, it is unlikely that the larvae are retained in the estuary. Instead, we conclude that U. vocator larvae are ‘exported’ by ebb tide currents to offshore waters, where salinity is higher and more constant and thus permits successful ontogenesis. Furthermore, the larval ‘export’ strategy would probably also reduce the risk of larval predation which is generally higher in inshore than in offshore waters (Morgan 1990, 1995). An investigation into the spatial and temporal distribution of U. vocator larvae in the field is now necessary to confirm the conclusion drawn from our laboratory experiment that U. vocator larvae at our study site develop offshore.
Our finding that ZI larvae of U. vocator survived for up to 3–7 days in salinities 0 and 5 before dying demonstrates that this specific zoeal stage has a considerable resistance to diluted media. Hence, it can tolerate a wide range of salinity and a remarkable hyper-/hypo-osmotic stress for a limited amount of time which would be important when dispersing from the estuarine to coastal waters. The same was found for the sympatric northern Brazilian mangrove crab species, U. cordatus, whose ZI larvae can also survive for up to 6 days in freshwater (Diele and Simith 2006). This physiological trait appears to be an evolutionary adaptation in species that reproduce in unpredictable environments with unstable salinity conditions, such as mangrove estuaries in northern Brazil during the wet season. Furthermore, the longer survival time in unfavourable low salinities gives the ZI larvae of decapod crabs more time to be transported by ebb tide currents from the estuary to more saline coastal waters allowing, this way, the moult to the subsequent zoeal stage (Anger et al. 1990). It is unlikely that lack of food caused the observed total mortality of U. vocator larvae in Sal 0, as fresh rotifers were frequently added. However, in decapod larvae food uptake and assimilation can be affected by reduced salinities, which thus may have an indirect impact on survival (Anger 2001, 2003).
By developing in saline offshore waters, hypo-osmotic stress can be avoided. However, the more advanced zoeal stages could probably develop in waters not too far off the coast as 88% still survived in salinity 15 in the laboratory. As U. vocator reproduces intensively during the rainy season, the adaptive resistance to intermediate salinities would be fundamental for larval survival in adjacent coastal waters. At our study site, the discharge of the Caeté river reaches 180 m3·s−1 during the rainy season (Lara and Dittmar 1999). Surface current speed during ebb tides ranges from 0.15–1.5 m·s−1 in tidal creeks (Cohen et al. 1999, Krumme and Saint-Paul 2003) and reaches 0.8 m·s−1 in nearshore waters (12 km from shore) (Cavalcante et al. 2010). Winds are strongest (6–8 m·s−1) between January and March (Allison et al. 1997). Such physical forces should transport crab larvae until at least 20 km away from the coast as has been observed by Diele (2000), investigating larval dispersion of U. cordatus in the same study area. The observed pattern of larval salinity tolerance in U. vocator reflects a limited capability of osmoregulation and an increasingly stenohaline response in the later zoeal stages, which appear to be strong osmoconformers. The ontogenetic changes in the osmoregulatory capability of the zoea larvae has been evidenced in many crabs and is typical for species with the larval ‘export’ strategy (Anger et al. 1990; Charmantier 1998; Anger and Charmantier 2000; Charmantier et al. 2002). In contrast, all larval stages of crab species with the larval ‘retention’ strategy are well adapted for development within their parental habitat where reduced and unstable salinities frequently occur (e.g. Rhithropanopeus harrisii: Costlow et al. 1966; Cronin 1982; A. miersii: Schuh and Diesel 1995a, b; Anger 1996; Sesarma curacaoense: Schuh and Diesel 1995c; Helice leachi, Mia and Shokita 2002). On the other hand, the tolerance of the larvae to low salinities may vary between populations of the same species that are geographically distant. In mangroves of other Brazilian coastal regions with less pronounced wet seasons and consequently more stable and higher salinity conditions, the larvae of U. vocator and also those from other co-occurring crabs may be less tolerant towards low salinity conditions. This could indicate a phenotypic plasticity permitting a better adaptation of the organisms to changes in local or regional salinity conditions. A comparative study is now under way to investigate the response of U. vocator larvae towards low salinities in different climatical and hydrological settings along the Brazilian coastline.
After ontogenesis in coastal or fully marine waters, megalopae of brachyuran crabs with larval ‘export’ strategy re-immigrate to the parental mangrove habitat and settle in specific sites attracted by natural physical and/or chemical stimuli (Anger 2001, 2006; Forward et al. 2001; Gebauer et al. 2003). When migrating upstream, they encounter low salinities, which they now are able to tolerate (Anger et al. 2006, 2008; Torres et al. 2006). Reduced salinity can in fact be important for guiding megalopae back to estuaries and/or stimulating settlement and metamorphosis. For U. vocator, we recently demonstrated that settlement in megalopae is triggered by environmental cues associated with mangrove estuaries (Simith et al. 2010). Whether reduced salinities also induce settlement in this species remains to be investigated.
In summary, our results show that salinity may exert a strong influence on the zoeal development of the fiddler crab U. vocator. Higher moulting rates and faster development occurred in intermediate and higher salinities (≥15), in contrast to the cultivation in Sal. 10. Moreover, extremely reduced salinities (0 and 5) were lethal for the ZI larvae, but they still managed to resist for a few days in these unfavourable conditions before dying. The developmental changes in larval salinity tolerance showed an increasingly stenohaline response of the zoeal stages which has important ecological implications. Ontogenetic migration from estuarine to offshore waters, with higher and more stable salinity conditions, seems to be a prerequisite for successful larval development in U. vocator. This way, by selection of a specific reproductive strategy and by means of ecological and physiological adaptations of the early life-history stages, the maintenance of viable populations in the unpredictable mangrove habitats in northern Brazil is assured.
References
Allison MA, Lee KT, Aller RC, Ogston AS (1997) Amazon sediment accumulation and mudbank genesis, Northern Amazona coast, Brazil. In: Flemming BW, Delafontaine M, Liebezeit G (eds) Muddy Coast. Berichte Forschungszentrum Terramare, no. 2, pp 14–15
Anger K (1991) Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Mar Ecol Prog Ser 72:103–110
Anger K (1996) Salinity tolerance of the larvae and first juveniles of a semiterrestrial grapsid crab, Armases miersii (Rathbun). J Exp Mar Biol Ecol 202:205–223
Anger K (2001) The biology of decapod crustacean larvae, crustacean issues. vol. 14. Lisse, The Netherlands: A. A. Balkema Publishers
Anger K (2003) Salinity as a key parameter in the larval biology of decapods crustaceans. Invertebr Reprod Dev 43(1):29–45
Anger K (2006) Contributions of larval biology to crustacean research: a review. Invertebr Reprod Dev 49(3):175–205
Anger K, Charmantier G (2000) Ontogeny of osmoregulation and salinity tolerance in a mangrove crab, Sesarma curacaoense (Decapoda: Grapsidae). J Exp Mar Biol Ecol 251:265–274
Anger K, Harms J, Montú M, Bakker C (1990) Effects of salinity on the larval development of a semiterrestrial tropical crab, Sesarma angustipes (Decapoda: Grapsidae). Mar Ecol Prog Ser 62:89–94
Anger K, Torres G, Giménez L (2006) Metamorphosis of a sesarmid river crab, Armases roberti: stimulation by adult odours versus inhibition by salinity stress. Mar Freshw Behav Phy 39(4):269–278
Anger K, Spivak E, Luppi T, Bas C, Ismael D (2008) Larval salinity tolerance of the South American salt-marsh crab, Neohelice (Chasmagnathus) granulata: physiological constraints to estuarine retention, export and reimmigration. Helgoland Mar Res 62:93–102
Cavalcante GH, Kjerfve B, Knoppers B, Feary DA (2010) Coastal currents adjacent to the Caeté Estuary, Pará Region, North Brazil. Estuarine Coastal Shelf Sci 88:84–90
Charmantier G (1998) Ontogeny of osmoregulation in crustaceans: a review. Invertebr Reprod Dev 33:177–190
Charmantier G, Charmantier-Daures M (1991) Ontogeny of osmoregulation and salinity tolerance in Cancer irroratus; elements of comparison with C. borealis (Crustacea, Decapoda). Biol Bull 180:125–134
Charmantier G, Charmantier-Daures M, Anger K (1998) Ontogeny of osmoregulation in the grapsid crab Amarses miersii (Crustacea: Decapoda). Mar Ecol Prog Ser 164:285–292
Charmantier G, Giménez L, Charmantier-Daures M, Anger K (2002) Ontogeny of osmoregulation, physiological plasticity and larval export strategy in the grapsid crab Chasmagnathus granulata (Crustacea, Decapoda). Mar Ecol Prog Ser 229:185–194
Cohen MCL, Lara RJ, Ramos JFF, Dittmar T (1999) Factors influencing the variability of Mg, Ca and K in waters of a mangrove creek in Bragança, North Brazil. Mangroves Salt Marshes 3:9–15
Costlow JD, Bookhout CG, Monroe R (1966) Studies on the larval development of the crab Rhitropanopeus harrisii (Gould). I. The effect of salinity and temperature on larval development. Physiol Zool 39:81–100
Cronin TW (1982) Estuarine retention of larvae of the mud crab Rhitropanopeus harrisii. Estuarine Coastal Shelf Sci 15:207–220
Diele K (2000) Life history and population structure of the exploited mangrove crab Ucides cordatus cordatus (L.) (Decapoda: Brachyura) in the Caeté estuary, North Brazil. Center for Tropical Marine Ecology, ZMT—Contribution 09. Bremen
Diele K, Simith DJB (2006) Salinity tolerance of northern Brazilian mangrove crab larvae, Ucides cordatus (Ocypodidae): necessity for larval export? Estuarine Coastal Shelf Sci 68:600–608
Diele K, Koch V, Abrunhosa FA, Lima JF, Simith DJB (2010) The brachyuran crab community of the Caeté Estuary, North Brazil: species richness, zonation and abundance. In: Saint-Paul U, Schneider H (eds) Mangrove dynamics and management in North Brazil. Ecological studies, vol 211. Springer, Berlin, Heidelberg, pp 251–263
Epifanio CE, Little KT, Rowe PM (1988) Dispersal and recruitment of fiddler crab larvae in the Delaware river estuary. Mar Ecol Prog Ser 43:181–188
Forward RB, Tankersley RA, Rittschof D (2001) Cues for metamorphosis of brachyuran crabs: an overview. Am Zool 41(5):1108–1122
Gebauer PI, Paschke K, Anger K (2003) Delayed metamorphosis in decapod crustaceans: evidence and consequences. Rev Chil Hist Nat 76:169–175
Giarrizzo T, Saint-Paul U (2008) Ontogenetic and seasonal shifts in the diet of Pemeceu sea catfish Sciades herzbergii (Siluriformes: Ariidae) from a macrotidal mangrove creek in the Curuçá estuary (North Brazil). Rev Biol Trop 56(2):861–873
Giménez L (2003) Potential effects of physiological plastic responses to salinities on population networks of the estuarine crab Chasmagnathus granulata. Helgoland Mar Res 56:265–273
Giménez L, Anger K (2001) Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J Exp Mar Biol Ecol 260:241–257
Giménez L, Anger K (2003) Larval performance in an estuarine crab, Chasmagnathus granulata, is a consequence of both larval and embryonic experience. Mar Ecol Prog Ser 249:251–264
Instituto Nacional de Meteorologia-INMET (1992) Normas Climatológicas. Brasília (DF). http://www.inmet.gov.br. Accessed in 2008
Koch V, Wolff M (2002) Energy budget and ecological role of mangrove epibenthos in the Caeté estuary, North Brazil. Mar Ecol Prog Ser 228:119–130
Koch V, Wolff M, Diele K (2005) Comparative population dynamics of four fiddler crabs (Ocypodidae, genus Uca) from a North Brazilian mangrove ecosystem. Mar Ecol Prog Ser 291:177–188
Krumme U, Saint-Paul U (2003) Observations of fish migration in a macrotidal mangrove channel in Northern Brazil using a 200-kHz split-beam sonar. Aquat Living Resour 16:175–184
Lara RJ, Dittmar T (1999) Nutrient dynamics in mangrove creek (North Brazil) during the dry season. Mangroves Salt Marshes 3:185–195
Luppi TA, Spivak ED, Bas CC (2003) The effects of temperature and salinity on larval development of Armases rubripes Rahtbun, 1897 (Brachyura, Grapsoidea, Sesarmidae), and the southern limit of its geographical distribution. Estuarine Coastal Shelf Sci 58:575–585
Melo GA (1996) Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro. São Paulo: (ed) Plêiade/FAPESP
Mia MDY, Shokita S (2002) Early life history of an estuarine grapsid crab, Helice leachi Hess. Indian J Fish 49:23–28
Morgan SG (1990) Impact of planktivorous fishes on dispersal, hatching and morphology of estuarine crab larvae. Ecology 71:1639–1652
Morgan SG (1995) Life and death in the plankton: larval mortality and adaptation. In: McEdward LR (ed) Ecology of marine invertebrate larvae. CRC press, Boca Raton, pp 279–321
O’Connor NJ, Epifanio CE (1985) The effect of salinity on the dispersal and recruitment of fiddler crab larvae. J Crustacean Biol 5:137–145
Rabalais NN, Cameron JN (1985) The effects of factors important in semi-arid environments on the early development of Uca subcylindrica. Biol Bull 168:147–160
Rieger PJ (1999) Desenvolvimento larval de Uca (Minuca) vocator (Herbst 1804) (Crustacea, Decapoda, Ocypodidae), em laboratório. Nauplius 7:1–37
Schuh M, Diesel R (1995a) Breeding in a rock pool: larvae of the semiterrestrial crab Armases [=Sesarma] miersii (Rathbun) Decapoda: Grapsidae) develop in a highly variable environment. J Exp Mar Biol Ecol 185:109–129
Schuh M, Diesel R (1995b) Effects of salinity, temperature, and starvation on the larval development of Armases (=Sesarma) miersii (Rathbun, 1897), a semiterrestrial crab with abbreviated development (Decapoda: Grapsidae). J Crustacean Biol 15:205–213
Schuh M, Diesel R (1995c) Effects of salinity and starvation on the larval development of Sesarma curacaoense de Man, 1892, a mangrove crab with abbreviated development (Decapoda: Grapsidae). J Crustacean Biol 15:645–654
Simith DJB, Diele K (2008) O efeito da salinidade no desenvolvimento larval do caranguejo-uçá, Ucides cordatus (Linnaeus, 1763) (Decapoda: Ocypodidae) no Norte do Brasil. Acta Amazon 38(2):345–350
Simith DJB, Diele K, Abrunhosa FA (2010) Influence of natural settlement cues on the metamorphosis of fiddler crab megalopae, Uca vocator (Decapoda: Ocypodidae). An Acad Bras Cienc 82(2):313–321
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. New York, WH Freeman and Company
Spivak ED, Cuesta JA (2009) The effect of salinity on larval development of Uca tangeri (Eydoux, 1835) (Brachyura: Ocypodidae) and new findings of the zoeal morphology. Sci Mar 73(2):297–305
Strathmann RR (1982) Selection for retention or export of larvae in estuaries. In: Kennedy VS (ed) Estuarine comparisons. Academic Press, New York, pp 521–535
Torres G, Anger K, Giménez L (2006) Effects of reduced salinities on metamorphosis of a freshwater-tolerant sesarmid crab, Armases roberti: Is upstream migration in the megalopa stage constrained by increasing osmotic stress? J Exp Mar Biol Ecol 338:134–139
Torres G, Giménez L, Anger K (2007) Effects of osmotic stress on crustacean larval growth and protein and lipid levels are related to life-histories: the genus Armases as a model. Comp Biochem Physiol 148:209–224
Torres G, Giménez L, Anger K (2008) Cumulative effects of low salinity on larval growth and biochemical composition in an estuarine crab, Neohelice granulata. Aquat Biol 2:37–45
Acknowledgments
We thank Luiz Paulo de Carvalho Melo and Marcus Alexandre Pires for their help in the laboratory during larval rearing and collections of ovigerous crabs. The present work was supported by the ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq), ‘Secretaria de Desenvolvimento, Ciência e Tecnologia’ (SEDECT), and ‘Fundação de Amparo à Pesquisa do Estado do Pará’ (FAPESPA). This paper resulted from cooperation between the Center for Tropical Marine Ecology (ZMT), Bremen, Germany and the Universidade Federal do Pará (UFPa), Belém, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Thiel.
Rights and permissions
About this article
Cite this article
de Jesus de Brito Simith, D., de Souza, A.S., Maciel, C.R. et al. Influence of salinity on the larval development of the fiddler crab Uca vocator (Ocypodidae) as an indicator of ontogenetic migration towards offshore waters. Helgol Mar Res 66, 77–85 (2012). https://doi.org/10.1007/s10152-011-0249-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-011-0249-0