Abstract
The community structure of the macrobenthic fauna was studied in the overall area of Laki Lagoon in September 1997 (salinity 32–35 psu) and monthly from February 1998 to February 1999 in the innermost part of the lagoon (salinity 0.1–6.8 psu). Community structure was analyzed by means of uni- and multivariate methods. In September 1997, the macrofauna in the outer part of the lagoon was characterized by a higher diversity and the occurrence of both lagoonal and marine species, and in the innermost part by a higher total abundance and the occurrence of a few lagoonal species. The combination of distance from the sea, depth, salinity and sediment organic matter correlated best with the spatial distribution pattern of the macrobenthic fauna. Community structure in the innermost part of the lagoon showed a seasonal periodicity. Species composition during spring 1998, at 0.1–2.0 psu, was similar to that in September 1997. During summer the macrobenthic fauna became impoverished, but recovered from late summer onwards. The salinity increase during summer (up to 5–7 psu) was followed by the appearance of marine species in the innermost part of the lagoon. Total abundance displayed a peak in late spring and a lower one in mid-autumn. The seasonal dynamics of the faunal assemblage was mainly governed by water temperature. Predation pressure by Atherina boyeri may have contributed to quantitative community changes during autumn.
Similar content being viewed by others
Introduction
Coastal lagoons, which are of considerable naturalistic and economic interest, are usually characterized by marked daily and seasonal fluctuations in environmental conditions (e.g. in salinity) depending on their geomorphology and hydrological regime. Additionally, in many cases, these coastal environments suffer severe dystrophic crises during summer, being followed by mortality events. Although coastal lagoons have been the subject of extensive studies, our knowledge of the factors determining the spatial and seasonal variation in their macrozoobenthic communities, especially in the microtidal Mediterranean Sea, is restricted and mainly related to abiotic rather than biotic factors (e.g. predation pressure). Useful information on the distribution and seasonal dynamics of the macrobenthic fauna in Mediterranean coastal brackish habitats has been given in some recent publications (e.g. Reizopoulou et al. 1996; Gouvis et al. 1997; Kevrekidis 1997; Lardicci et al. 1997; Tagliapietra et al. 1998; Arvanitidis et al. 1999; Bachelet et al. 2000; Koutsoubas et al. 2000; Mistri et al. 2001; Mistri 2002). However, very little information exists on the seasonal dynamics of macrozoobenthic communities in low-salinity parts of Mediterranean lagoons.
Laki Lagoon, one of the three lagoons of the Evros Delta (northern Aegean), which is protected as a wetland of international value according to the Ramsar Convention, is of notable natural history and recreational interest. Nevertheless, information on the macrobenthic fauna of Laki Lagoon is mainly limited to polychaetes, amphipods and molluscs (Kevrekidis and Koukouras 1988; Gouvis and Koukouras 1993; Kevrekidis et al. 1996; Gouvis et al. 1998).
The main goal of this study is a better understanding of the structure and function of coastal brackish ecosystems through (1) the description of the distribution of the macrobenthic fauna in Laki Lagoon and of the seasonal variation in macrozoobenthic community structure and dynamics at very low salinities, and (2) the investigation of the key variables affecting this spatial and seasonal variation.
Methods
Study area
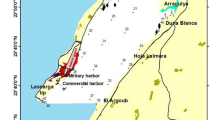
Evros Delta is located at the NE end of the Aegean Sea (Fig. 1). Fresh water flows into the delta area primarily from the eastern branch of the Evros river; additional fresh water enters the delta area through the western branch of the Evros and the streams Mikri Maritsa and Loutron, usually from autumn to early summer. Three islets and some lagoons have been formed in the delta area. Laki Lagoon, occupying an area of about 100 ha, communicates with the sea via two openings, both situated on the western side of the lagoon (Fig. 1).
Sampling and laboratory techniques
Samples of the benthic fauna were collected in September 1997 at sampling sites LA1 and LA2 located on a transect across the innermost, northern part of Laki; at sampling sites LC1, LC2 and LC3 located on a transect across the southernmost part of the lagoon; and at sampling site LB located at the central part of the lagoon (Fig. 1). At each site, two random replicate units were taken using a van Veen-type special sampler with handles (Larimore 1970); the sampler covered a surface of 400 cm2 (20×20 cm2) and penetrated to a depth of 20 cm. The samples were sieved on a 0.5-mm mesh sieve and the animals were kept in 5% formalin solution. Depth and water salinity near the bottom were also measured. Sediment samples were taken using a small corer for particle size analysis and estimation of the amount of organic matter.
Monthly samples were collected from February 1998 to February 1999 at station LA1 (Fig. 1). Four random replicate units were taken using the modified van Veen grab. The samples were sieved through a 0.5-mm screen. Sediment samples were taken using a small corer. Depth, salinity, temperature and dissolved O2 of the water near the bottom were also measured. Additionally, two samples of epibenthic decapods and small fish were taken using a special net (with a 40×40 cm opening) which was pulled on the sediment for a distance of 10 m.
Sediment analysis and estimation of organic matter were made according to the methods described by Buchanan (1984). All animals were identified to the lowest possible taxon and counted.
Data analysis
Macrobenthic community structure was analyzed by the total number of individuals (n), number of species (S), and Margalef’s species richness (d), Shannon–Wiener species diversity (H′) (natural logarithm) and evenness (J) indices.
The spatial and seasonal macrofaunal patterns were tested using the Analysis of Similarities (ANOSIM) test. Non-metric multidimensional scaling (MDS) was used to investigate faunal similarities among samples (data transformed to fourth root). Species responsible for similarities and for differences between sample groups were investigated using the similarity percentages routine (SIMPER) (Clarke 1993). Environmental variables which correlated best with the multivariate pattern of the macrobenthic community were identified by means of harmonic Spearman coefficient, ρw (BIO–ENV analyses) (Clarke and Ainsworth 1993). Spearman’s rank correlation coefficient was applied to identify the highly correlated environmental variables. The PRIMER package developed at Plymouth Marine Laboratory was used.
Results
The environment
Abiotic variables
The distance of the sampling stations from the sea and the values of abiotic variables of water and sediment at these stations in September 1997 are shown in Table 1. Station LB and transect LC were located closer to the sea (Fig. 1, Table 1). Transect LA was characterized by shallow waters, while along transect LC sediments were coarser and sediment organic matter content was lower (Table 1). Salinity near the bottom varied between 32.2 psu and 35.1 psu throughout the lagoon (Table 1).
An increased freshwater inflow during winter and spring 1998, following a period of intense rainfall on the Evros catchment area, resulted in a sharp decline in salinity in Laki. Salinity near the bottom was close to 0 psu in February/March 1998; it increased during spring, peaked in late summer (6.8 psu) and then gradually decreased until February 1999 (0.2 psu) (Fig. 2). The values of abiotic factors of water and sediment at station LA1 from February 1998 to February 1999 are shown in Table 2. Depth varied between 5 cm and 50 cm. Water temperature near the bottom was lowest in February/March 1998 (~10.5°C) and from November 1998 to January 1999 (6.7–9.6°C), and highest in July (25.9°C) (Table 2; Fig. 2). Dissolved oxygen showed relatively high values in early spring, autumn and winter and was minimal in June (5.3 mg/l). The sediment consisted of very fine sand almost throughout the sampling period; sediment organic matter showed comparatively high values from July to October (1.17–1.66%) and in February 1999 (1.48%) (Table 2). These values were much lower than those reported from several other Mediterranean lagoons (e.g. Reizopoulou et al. 1996; Lardicci et al. 1997; Tagliapietra et al. 1998).
Biotic variables
Macroalgae were present in the innermost part of Laki Lagoon (station LA1) (Fig. 1) throughout the study period (February 1998–February 1999): their biomass (as total algal dry weight) varied seasonally, showing main peaks in August (135.43 g m−2) and in November (101.20 g m−2) (unpublished data; see also Table 3).
Four species of decapods and five species of small fish were found in the monthly samples taken with the special net at station LA1 from February 1998 to February 1999. The monthly variation in the number of individuals/10 m2 is given in Table 3. Most of them were mainly found in summer and/or autumn; Atherina boyeri was collected in September and in November.
Faunal composition
A total of 25 macrobenthic invertebrates was found along transects LA and LC and at station LB in September 1997. Sixteen of these species displayed a cumulative mean dominance of 99% (Table 4). The polychaete Streblospio shrubsolii and Tubificidae were found at all stations showing a cumulative mean dominance of 48% (Table 4). The amphipods Corophium orientale and Gammarus aequicauda, the polychaete Capitomastus minimus and the gastropod Ventrosia maritima also showed a remarkable mean dominance although they were less frequently collected (Table 4).
Nineteen taxa were collected at station LA1 throughout the annual cycle (February 1998–February 1999). Thirteen of these taxa showed a cumulative mean monthly dominance of 99.6%; four species were constantly found throughout the study period (Table 5). The amphipod C. orientale dominated the assemblage, showing a mean monthly dominance of 54.6%. The polychaetes S. shrubsolii and Hediste diversicolor and the bivalve Abra ovata displayed a more or less remarkable mean monthly dominance. Four taxa (Tubificidae, V. maritima, G. aequicauda and Cerastoderma glaucum) were frequently found (in 10 or 9 months); however, only the first two taxa showed a notable mean monthly dominance.
Structural analyses
Univariate analysis
The major biological parameters (total density n, number of species S, species richness d, species diversity H′ and evenness J) at each station in September 1997 are given in Table 6. The n showed its highest values on transect LA, especially at station LA1, and was minimal at station LB; S and d had comparatively higher values at stations LC1 and LC2 and the lowest values at station LB. H′ had its highest values on transect LC and was minimal at station LB, as well; J showed its highest value at station LC3.
Total macrobenthic density at station LA1 increased sharply from February/March 1998 (mean values 8,544 and 5,406 individuals m−2, respectively) to May (22,225 individuals m−2); then it decreased sharply to September (2,906 individuals m−2) (Fig. 3). Density temporarily increased in October (9,463 individuals m−2) and then again gradually from November to January/February 1999 (7,231–7,719 individuals m−2). Mean value of S ranged between 6.75 and 7.75 from February 1998 to July (with the minimum in July), between 7.5 and 9 from August to November, and between 10.25 and 11.75 from December 1998 to February 1999 (Fig. 4). Mean value of d varied between 0.605 and 0.748 from February 1998 to July (with the minimum in July), between 0.799 and 1.007 from August to November, and between 1.041 and 1.249 from December 1998 to February 1999 (Fig. 4). Mean values of H′ and J fluctuated between 0.787 and 1.7 and between 0.363 and 0.804, respectively, being lowest from May to July and in October (Fig. 4). A temporary sharp increase in abundance of C. orientale contributed to the low values of H′ and J in October.
Multivariate analysis
Two significantly distinct groups were formed by the samples collected at the sampling stations in September 1997 (ANOSIM, global R=0.89, P<0.001). The first group was formed by the samples collected in the outer, southernmost part of the lagoon (stations LC1–LC3) along with a sample collected at station LB, and the second group by the samples collected in the shallower, innermost northern part of the lagoon (stations LA1, LA2). The MDS ordination plot of the samples collected at the sampling stations in September 1997 is shown in Fig. 5.
Four taxa (S. shrubsolii, Tubificidae, Aricidea cerrutii and Glycera tridactyla) contributed >65% to the similarity in the first sample group, and five species (C. orientale, V. maritima, A. ovata, S. shrubsolii and C. glaucum) in the second group (SIMPER) (Table 7). The differences between these groups were mainly due to (1) the exclusive occurrence of three species in the first group (A. cerrutii, G. tridactyla, C. minimus) and of two species in the second one (V. maritima, H. diversicolor), and (2) the higher average density of four taxa (C. orientale, A. ovata, Tubificidae, C. glaucum) in the second group (SIMPER, cut-off 65%) (Table 8). A sample collected at station LB (sample1 LB) was not grouped (Fig. 5); macrofauna of this sample was extremely poor (only two taxa in extremely low densities) (Table 8).
Four significantly distinct groups were formed by the monthly samples (ANOSIM, global R=0.815, P<0.001) corresponding to different periods of the year. The first group included the samples of February 1998 to May, the second those of June and July, the third those of August to October, and the fourth those of November to February 1999. The MDS ordination plot of the monthly samples at station LA1 is shown in Fig. 6.
The average densities of the species which contributed >65% to the similarity in each monthly sample group (SIMPER) are given in Table 9; it can be shown that almost the same taxa contributed to the similarity in each group. The average densities of the species which were responsible for the differences between the successive monthly sample groups (cut-off 65%; SIMPER) are given in Table 10. The differences between late winter/spring samples and those of summer were mainly caused by the disappearance of some taxa (Tubificidae, Cumacea), the decline in density of some molluscs (V. maritima, A. ovata) and the increase in density of the epibenthic amphipod G. aequicauda and the “enriched opportunist” polychaete S. shrubsolii in summer. The differences between summer and late summer/autumn samples were primarily due to the appearance of some taxa (Tubificidae, C. glaucum, Microprotopus maculatus), the increase in density of A. ovata and the decline in that of V. maritima during late summer/autumn. The differences between late summer/autumn samples and those of late autumn/winter were mainly caused by the appearance of some polychaetes (Polydora ciliata, G. tridactyla, A. cerrutii), the increase in density of some taxa (Tubificidae, S. shrubsolii) and the decrease in that of G. aequicauda, V. maritima and C. glaucum during late autumn/winter (Table 10).
Associated environmental variables
The highest values of the harmonic Spearman rank coefficient (ρw), deriving from the performance of the BIO–ENV analysis, are given in Table 11. From the examined abiotic variables (Table 1), the set of distance from the sea, depth, salinity and sediment organic matter correlated best with the distribution pattern of the macrobenthic community; the set of distance from the sea and salinity showed the next highest value of ρw (Table 11). From a total of 16 abiotic and biotic variables (Tables 2, 3), water temperature correlated best with the monthly variation in the macrobenthic community structure at station LA1 (Table 11). The set of water temperature with the abundance of A. boyeri showed the next highest value of ρw (Table 11).
Discussion
The distribution of the macrobenthic fauna in Laki seems to conform to the “confinement” theory proposed by Guelorget and Perthuisot (1992). The outer areas of Laki lagoon, close to the lagoon mouths, showed a different faunal composition compared to the innermost part of the lagoon in early autumn at salinities of 32–35 psu, indicating a biological zonation.
Macrofauna in the outer part of Laki was characterized by a higher diversity and the occurrence of both brackish-water taxa (e.g. S. shrubsolii, tubificids, C. orientale, A. ovata) and marine species (e.g. A. cerrutii, G. tridactyla, C. minimus). Most of these marine species have also previously been found in brackish habitats (e.g. Picard 1965; Fèbvre 1968; Nicolaidou and Pitta 1986; Gravina et al. 1988; Nicolaidou and Papadopoulou 1989; Thiermann et al. 1997; Clarke and Warwick 1998; Gouvis et al. 1998). According to Cognetti and Maltagliati (2000) brackish habitats which communicate directly with the sea are usually colonized by euryhaline marine species, whose populations have developed a tolerance to unpredictable changes in habitat, and by stenohaline marine species which have developed euryhaline populations.
The macrobenthic assemblage in the innermost part of Laki was characterized by a higher total density and the occurrence of a few species which are exclusive inhabitants of coastal brackish habitats. These species have marine (e.g. S. shrubsolii, C. orientale, V. maritima, A. ovata, C. glaucum, H. diversicolor) or limnic origin (tubificid oligochaetes) (e.g. Diviacco 1983; Gravina et al. 1988; Kevrekidis and Koukouras 1988; Sarda and Martin 1993; Kevrekidis et al. 1996; Gouvis et al. 1998). Species composition was quite similar to that in other brackish habitats of the Evros Delta which have a moderate access to the sea (e.g. Kevrekidis 1997) and assemblage characteristics (a limited number of species, a strong dominance in abundance by a few of these species, relatively low diversity) were similar to those previously reported for other Mediterranean brackish habitats (e.g. Reizopoulou et al. 1996; Mistri et al. 2001). Cognetti and Maltagliati (2000) stated that the same euryhaline species usually constitute a typical brackish community, particularly in habitats in the same biogeographical region; their occurrence depends on basin area and on their individual tolerance to variations in some abiotic factors other than salinity (e.g. temperature, oxygen availability, substrate).
The set of distance from the sea, depth, salinity and organic matter of the sediment was found to correlate best with the spatial distribution pattern of the macrobenthic fauna in Laki. This suggests that confinement results from the combined effects of these variables. Koutsoubas et al. (2000) suggested that confinement is determined by a few variables, the extreme values of which produce a gradient along a lagoon and act as thresholds to species distribution.
Macrozoobenthic community structure in the innermost part of Laki lagoon showed seasonal variation. Its dynamics was mainly governed by water temperature. Temperature, along with photoperiod, provide a seasonal cue for the timing of reproduction of macrozoobenthic species and therefore for community changes (e.g. De March 1977; Whitlatch 1977; Moore 1981). The available information on the life history of some of the frequent species in the assemblage, such as A. ovata and G. aequicauda (e.g. Kevrekidis and Koukouras 1988, 1989, 1992), indicates that the observed seasonal variation in density of these species is mainly a result of the timing of their reproductive cycle.
The macrobenthic fauna in the innermost part of Laki lagoon was composed of only a few brackish-water species during the late winter/spring 1998 period at salinities of 0.1–2 psu. Extremely low salinities over a long period were possibly responsible for the decline in most community indices during spring. Species composition (C. orientale, S. shrubsolii, H. diversicolor, V. maritima, A. ovata, G. aequicauda, etc.) was quite similar to that in early autumn 1997 at 32–35 psu. This, along with the increase in total density during spring, reveals the euryhaline character of most community species. Most of the collected species have rarely been reported with this high level of density from habitats sustaining such low salinities for long periods (e.g. Cunha and Moreira 1995).
Some kind of stress most probably resulted in the impoverishment of the macrobenthic fauna during summer. That impoverishment was characterized by the disappearance of some taxa (Tubificidae, Cumacea) or the decline in density of some species (A. ovata, V. maritima); total density also decreased and community indices showed comparatively low values during summer. This disturbance was probably due to a dystrophic episode. The lowest dissolved oxygen concentrations during daytime (5.3 mg/l) were observed in June, probably due to a rapid decomposition of macroalgae. Moreover, anoxic/hypoxic conditions possibly occurred from late night to early morning during summer, when respiratory oxygen consumption by the increased number of macroalgae was probably not compensated for by photosynthetic oxygen production (e.g. Cunha and Moreira 1995; Lardicci et al. 1997; Tagliapietra et al. 1998).
The recovery of the macrofauna in the innermost part of Laki lagoon started in late summer. Furthermore, marine species (M. maculatus, P. ciliata, G. tridactyla, A. cerrutii) occurred in the innermost part of the lagoon during late summer, autumn and winter, probably as a result of immigration or transportation of larvae from the outer part of the lagoon or from marine environments surrounding the lagoon. That occurrence followed, with a time lag, the increase in salinity above 5 psu during summer and autumn, and was also reflected in an additional increase in community indices. Most of the aforementioned marine species were also previously found in brackish habitats (e.g. Gravina et al. 1988; Kevrekidis and Koukouras 1988; Nicolaidou and Papadopoulou 1989; Thiermann et al. 1997; Gouvis et al. 1998; Mistri et al. 2001), while the 5 psu salinity limit is considered as the critical threshold for the occurrence of marine species in these habitats (Cognetti and Maltagliati 2000).
The occurrence of marine species in the innermost part of Laki suggests a shift of the limits of the lagoonal zones during the recovery phase. Koutsoubas et al. (2000) also observed that the limits of the zones in another Mediterranean lagoon (Gialova Lagoon, Ionian Sea) show seasonal shifts; they suggested that the gradient produced by the extreme values of a few environmental variables along a lagoon disappears during the recovery phase.
Predation pressure by A. boyeri possibly contributed to the decline in density of some invertebrates (e.g. G. aequicauda, V. maritima, C. glaucum) during late autumn and winter and in total density in September and November as well. It is known that this small fish, which was collected only in September and November in Laki, feeds on macrobenthic invertebrates (Quignard and Pras 1986). Many authors have stressed the role of predation by fish and decapods (top-down control) in regulating the dynamics of macrobenthic fauna (e.g. Wiltse et al. 1984; Aarnio and Bonsdorff 1993; Sarda et al. 1996). However, the abundance of no other epibenthic decapod or small fish displayed a strong correlation with community structure, although the diet of most of them is known to include macrobenthic invertebrates (e.g. Muus 1967; Scalera Liaci et al. 1982; Cottiglia et al. 1983a, 1983b; Khoury 1984; Maugé 1986; Kevrekidis et al. 1990). The increased number of macroalgae during summer and autumn, when both epibenthic predators are most abundant and their feeding activity, especially in summer, is probably high, may offer macrobenthic invertebrates a refuge from predation (Arias and Drake 1994).
The abundance of the macrobenthic invertebrates in the innermost part of Laki lagoon displayed a seasonal variation with a peak in late spring and a lower one in mid autumn. As is typical of Mediterranean coastal lagoons, the macrofauna is exposed to extreme environmental conditions during summer, and macroinvertebrate abundance thus usually shows a seasonal variation with a peak in winter or spring (e.g. Guelorget and Michel 1979a) and sometimes a second one in autumn (e.g. Guelorget and Michel 1979a, 1979b; Gouvis et al. 1997; Mistri et al. 2001). A seasonal variation of total abundance with a peak in mid or late autumn has also been observed (e.g. Guelorget and Michel 1979a; Kevrekidis 1997).
References
Aarnio K, Bonsdorff E (1993) Seasonal variation in abundance and diet of the sand goby Pomatoschistus minutus (Pallas) in a northern Baltic Archipelago. Ophelia 37:19–30
Arias AM, Drake P (1994) Structure and production of the benthic macroinvertebrate community in a shallow lagoon in the Bay of Cádiz. Mar Ecol Prog Ser 115:151–167
Arvanitidis C, Koutsoubas D, Dounas C, Eleftheriou A (1999) Annelid fauna of a Mediterranean lagoon (Gialova Lagoon, south-west Greece): community structure in a severely fluctuating environment. J Mar Biol Assoc UK 79:849–856
Bachelet G, Montaudouin De X, Auby I, Labourg PJ (2000) Seasonal changes in macrophyte and macrozoobenthos assemblages in three coastal lagoons under varying degrees of eutrophication. ICES J Mar Sci 57:1495–1506
Buchanan JB (1984) Sediment analysis. In: Holme NA, McIntyre AD (eds) Methods for the study of marine benthos. IBP Handbook No. 16, Oxford, pp 41–65
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Warwick RM (1998) Quantifying structural redundancy in ecological communities. Oecologia 113:278–289
Cognetti G, Maltagliati F (2000) Biodiversity and adaptive mechanisms in brackish water fauna. Mar Pollut Bull 40:7–14
Cottiglia M, Tagliasacchi Masala ML, Serra E (1983a) Relations trophiques dans une lagune littoral tyrrhénienne. 1 - Réseaux basés sur le phytoplancton, le tripton et les dépouilles animales. Rapp Comm int Mer Médit 28:147–149
Cottiglia M, Tagliasacchi Masala ML, Serra E (1983b) Relations trophiques dans une lagune littoral tyrrhénienne. 2- Réseaux basés sur la phytobenthos et les détritus. Rapp Comm int Mer Médit 28:151–153
Cunha MR, Moreira MH (1995) Macrobenthos of Potamogeton and Myriophyllum beds in the upper reaches of Canal de Mira (Ria de Aveiro, NW Portugal): community structure and environmental factors. Neth J Aquat Ecol 29:377–390
De March BGE (1977) The effects of photoperiod and temperature on the induction and termination of reproductive resting stage in the freshwater amphipod Hyalella azteca (Saussure). Can J Zool 55:1595–1600
Diviacco G (1983) Distribution of the crustacean amphipods in the East Tyrrhenian lagoons. Rapp Comm int Mer Médit 28:315–318
Fèbvre J (1968) Etude de bionomique des substrats meubles de l’ etang de Berre. Rec Trav Sta Mar Endoume 44:298–355
Gouvis N, Koukouras A (1993) Macrozoobenthic assemblages of the Evros Delta (North Aegean Sea). Int Revue ges Hydrobiol 78:59–82
Gouvis N, Kevrekidis T, Koukouras A (1997) Temporal changes of a macrobenthic assemblage in Evros Delta (North Aegean Sea). Int Revue ges Hydrobiol 82:67–80
Gouvis N, Kevrekidis T, Koukouras A, Voultsiadou E (1998) Bionomy of macrobenthic polychaetes in the Evros Delta (North Aegean Sea). Int Rev Hydrobiol 83:145–161
Gravina MF, Ardizzone GD, Giangrande A (1988) Selecting factors in polychaete communities of central Mediterranean coastal lagoons. Int Revue ges Hydrobiol 73:465–476
Guelorget O, Michel P (1979a) Les peuplements benthiques d’ un étang littoral Languedocien, l’ étang du Prévost (Hérault). 1. Étude quantitative de la macrofaune des vases. Téthys 9:49–64
Guelorget O, Michel P (1979b) Les peuplements benthiques d’ un étang littoral Languedocien, l’ étang du Prévost (Hérault). 2. Étude quantitative de la macrofaune des sables. Téthys 9:65–77
Guelorget O, Perthuisot JP (1992) Paralic ecosystems. Biological organization and functioning. Vie Milieu 42:215–251
Kevrekidis T (1997) Seasonal variation of the macrobenthic fauna in an isolated area of the Evros Delta (North Aegean Sea). Isr J Zool 43:243–255
Kevrekidis T, Koukouras A (1988) Bionomy of the amphipods in the Evros Delta (North Aegean Sea). PSZNI: Mar Ecol 9:199–212
Kevrekidis T, Koukouras A (1989) Life cycle and reproduction of Gammarus aequicauda (Crustacea: Amphipoda) in the Evros Delta (NE Greece). Isr J Zool 35:137–149
Kevrekidis T, Koukouras A (1992) Population dynamics, growth and productivity of Abra ovata (Mollusca, Bivalvia) in the Evros Delta (North Aegean Sea). Int Revue ges Hydrobiol 77:291–301
Kevrekidis T, Gouvis N, Koukouras A (1996) Bionomy of macrobenthic molluscs in Evros Delta (North Aegean Sea). Int Revue ges Hydrobiol 81:455–468
Kevrekidis T, Kokkinakis A, Koukouras A (1990) Some aspects of the biology and ecology of Knipowitschia caucasica (Teleostei: Gobiidae) in the Evros Delta (North Aegean Sea). Helgolander Meeresunters 44:173–187
Khoury C (1984) Ethologies alimentaires de quelques espèces de poisons de l’herbier de Posidonies du Parc National de Port-Cros. In: Boudouresque CF, Jeudy de Grissac A, Olivier J (eds) International workshop Posidonia Oceanica Beds. GIS Posidonie Publications, France 1, pp 335–347
Koutsoubas D, Dounas C, Arvanitidis C, Kornilios S, Petihakis G, Triantafyllou G, Eleftheriou A (2000) Macrobenthic community structure and disturbance assessment in Gialova Lagoon, Ionian Sea. ICES J Mar Sci 57:1472–1480
Lardicci C, Rossi F, Castelli A (1997) Analysis of macrozoobenthic community structure after severe dystrophic crises in a Mediterranean coastal lagoon. Mar Pollut Bull 34:536–547
Larimore WR (1970) Two shallow-water bottom samplers. The Progressive Fish Culturist 116–118
Maugé LA (1986) Gobiidae. In: Daget J, Gosse J-P, Thys van den Audenaerde DFE (eds) Check-list of the freshwater fishes of Africa (CLOFFA), vol 2. ISNB, Brussels; MRAC, Tervuren and ORSTOM, Paris. pp 358–388
Mistri M (2002) Persistence of benthic communities: a case study from the Valli di Comacchio, a Northern Adriatic lagoonal ecosystem (Italy). ICES J Mar Sci 59:314–322
Mistri M, Rossi R, Fano EA (2001) Structure and secondary production of a soft bottom macrobenthic community in a brackish lagoon (Sacca di Goro, north-eastern Italy). Estuar Coast Shelf Sci 52:605–616
Moore PG (1981) The life histories of the amphipods Lembos websteri Bate and Corophium bonnellii Milne Edwards in kelp holdfasts. J Exp Mar Biol Ecol 49:1–50
Muus BJ (1967) The fauna of Danish estuaries and lagoons. Medd Dan Fisk Havunders 5:1–316
Nicolaidou A, Papadopoulou KN (1989) Factors affecting the distribution and diversity of polychaetes in Amvrakikos Bay, Greece. PSZNI: Mar Ecol 10:193–204
Nicolaidou A, Pitta P (1986) Fauna associated with marine plants in a brackish lagoon of Amvrakikos bay. Biol Gallo-hellenica 12:141–147
Picard J (1965) Recherches qualitatives sur les biocoenoses marines des substrats meubles dragables de la region Marseillaise. Rec Trav Sta Mar Endoume 52:1–160
Quignard J-P, Pras A (1986) Atherinidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (eds) Fishes of the North-eastern Atlantic and the Mediterranean, vol 3. UNESCO, Paris, pp 1207–1210
Reizopoulou S, Thessalou-Legaki M, Nicolaidou A (1996) Assessment of disturbance in Mediterranean lagoons: an evaluation of methods. Mar Biol 125:189–197
Sarda R, Martin D (1993) Populations of Streblospio (Polychaeta: Spionidae) in temperate zones: demography and production. J Mar Biol Assoc UK 73:769–784
Sarda R, Valiela I, Foreman K (1996) Decadal shifts in a salt marsh macroinfaunal community in response to sustained long-term experimental nutrient enrichment. J Exp Mar Biol Ecol 205:63–81
Scalera Liaci L, Piscitelli G, Sciscioli M (1982) Ricerche sull’ alimentazione naturale di Penaeus kerathurus (Forskal, 1775). Oebalia 8:15–29
Tagliapietra D, Pavan M, Wagner C (1998) Macrobenthic community changes related to eutrophication in Palude della Rosa (Venetian Lagoon, Italy). Estuar Coast Shelf Sci 47:217–226
Thiermann F, Akoumianaki I, Hughes JA, Giere O (1997) Benthic fauna of a shallow-water gaseohydrothermal vent area in the Aegean Sea (Milos, Greece). Mar Biol 128:149–159
Whitlatch RB (1977) Seasonal changes in the community structure of the macrobenthos inhabiting the intertidal sand and mud flats of Barnstable Harbor, Massachusetts. Biol Bull 152:275–294
Wiltse WI, Foreman KH, Teal JM, Valiela I (1984) Effects of predators and food resources on the macrobenthos of salt marsh creeks. J Mar Res 42:923--942
Acknowledgements
This study was conducted in the Laboratory of Environmental Research and Education of Democritus University of Thrace. Many thanks go to Ms T. Boubonari and Ms V. Kalpia for sampling and laboratory assistance. The authors are grateful to Prof. Dr. Thomas Wilke (Animal Ecology and Systematics, Justus Liebig University Giessen, Germany) for the identification of the gastropod Ventrosia maritima using DNA sequencing data, and to Dr. C. Arvanitidis (Institute of Marine Biology of Crete, Hellas) for assistance with the PRIMER package, for the long discussion and the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke
Rights and permissions
About this article
Cite this article
Mogias, A., Kevrekidis, T. Macrozoobenthic community structure in a poikilohaline Mediterranean lagoon (Laki Lagoon, northern Aegean). Helgol Mar Res 59, 167–176 (2005). https://doi.org/10.1007/s10152-004-0215-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-004-0215-1