Abstract
Background
Glioma is the most common intracranial malignancy in adults with a high degree of malignancy and poor prognosis, which is largely attributed to the existence of glioma stem cells (GSCs). Previous evidence indicated that the matrix metalloproteinase ADAMTS1 was implicated in the process of tumor invasion, but the involvement of ADAMTS1 in glioma malignant invasion remains poorly understood.
Methods
The expression and prognosis values of ADAMTS1 were investigated in patients with glioma based on ONCOMINE and GEPIA databases. ADAMTS1 expression of different malignancy grade tissues was determined by immunohistochemistry. The effects of ADAMTS1 on cell proliferation and invasion were determined by clone formation assay and Transwell migration assay. The animal experiment was performed in an intracranial orthotopic xenograft model by knockout of ADAMTS1. Stemness properties and Notch1-SOX2 pathway were examined in stable ADAMTS1 knockdown GSCs.
Results
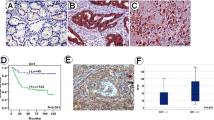
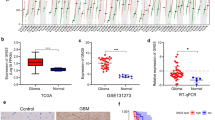
The expression levels of ADAMTS1 were significantly higher in glioma tissues and significantly correlated with the grade of malignancy and prognosis of glioma. Elevated ADAMTS1 expression was associated with SOX2, N-cadherin and the resistance of chemoradiotherapy of glioma patients. ADAMTS1 knockout suppressed the intracranial orthotopic xenograft growth and prolonged the survival of xenograft mice in vivo. Mechanistically, we found a blockade of the migration and invasiveness of GSCs and the expression levels of Notch1 and SOX2 in absence of ADAMTS1.
Conclusion
As a biomarker for prediction of prognosis, ADAMTS1 may affect the invasive phenotype of GSCs by regulating Notch1-SOX2 signaling pathway, thereby promoting the invasive growth of glioma.






Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lara-Velazquez M, Al-Kharboosh R, Jeanneret S et al (2017) Advances in brain tumor surgery for glioblastoma in adults. Brain Sci 7(12):166. https://doi.org/10.3390/brainsci7120166
Eisemann T, Costa B, Strelau J et al (2018) An advanced glioma cell invasion assay based on organotypic brain slice cultures. BMC Cancer 18(1):103. https://doi.org/10.1186/s12885-018-4007-4
Neman J, Jandial R (2010) Decreasing glioma recurrence through adjuvant cancer stem cell inhibition. Biol: Targets Ther 4:157–162. https://doi.org/10.2147/btt.s9497
Huang Q, Zhang QB, Dong J et al (2008) Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC Cancer 8:304. https://doi.org/10.1186/1471-2407-8-304
Liebelt BD, Shingu T, Zhou X et al (2016) Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int 2016:7849890. https://doi.org/10.1155/2016/7849890
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374. https://doi.org/10.1038/nrc1075
Mentlein R, Hattermann K, Held-Feindt J (2012) Lost in disruption: role of proteases in glioma invasion and progression. Biochim Biophys Acta 182(2):178–185. https://doi.org/10.1016/j.bbcan.2011.12.001
Kuno K, Kanada N, Nakashima E et al (1997) Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem 272(1):556–562. https://doi.org/10.1074/jbc.272.1.556
Kuno K, Terashima Y, Matsushima K (1999) ADAMTS-1 is an active metalloproteinase associated with the extracellular matrix. J Biol Chem 274(26):18821–18826. https://doi.org/10.1074/jbc.274.26.18821
Tyan SW, Hsu CH, Peng KL et al (2012) Breast cancer cells induce stromal fibroblasts to secrete ADAMTS1 for cancer invasion through an epigenetic change. PLoS ONE 7(4):e35128. https://doi.org/10.1371/journal.pone.0035128
Wang W, He L, Lin Z (2009) Biological characteristics of glioma stem cells. Chin J Neurosurg Dis Res 8:283–285. https://doi.org/10.3969/j.issn.1671-2897.2009.03.028
Wang S, Liu Y, Zhao G et al (2018) Postnatal deficiency of ADAMTS1 ameliorates thoracic aortic aneurysm and dissection in mice. Exp Physiol 103(12):1717–1731. https://doi.org/10.1113/ep087018
Ho IAW, Shim WSN (2017) Contribution of the microenvironmental niche to glioblastoma heterogeneity. Biomed Res Int 2017:9634172. https://doi.org/10.1155/2017/9634172
Hira VVV, Aderetti DA, van Noorden CJF (2018) Glioma stem cell niches in human glioblastoma are periarteriolar. J Histochem Cytochem: Off J Histochem Soc 66(5):349–358. https://doi.org/10.1369/0022155417752676
Mao DD, Gujar AD, Mahlokozera T et al (2015) A CDC20-APC/SOX2 signaling axis regulates human glioblastoma stem-like cells. Cell Rep 11(11):1809–1821. https://doi.org/10.1016/j.celrep.2015.05.027
Fan X, Khaki L, Zhu TS et al (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells (Dayton, Ohio) 28(1):5–16. https://doi.org/10.1002/stem.254
Cristofaro I, Alessandrini F, Spinello Z et al (2020) Cross interaction between M2 muscarinic receptor and Notch1/EGFR pathway in human glioblastoma cancer stem cells: Effects on cell cycle progression and survival. Cells. https://doi.org/10.3390/cells9030657
Sun Z, Wang L, Zhou Y et al (2020) Glioblastoma stem cell-derived exosomes enhance stemness and tumorigenicity of glioma cells by transferring Notch1 protein. Cell Mol Neurobiol 40(5):767–784. https://doi.org/10.1007/s10571-019-00771-8
Wang J, Xu SL, Duan JJ et al (2019) Invasion of white matter tracts by glioma stem cells is regulated by a NOTCH1-SOX2 positive-feedback loop. Nat Neurosci 22(1):91–105. https://doi.org/10.1038/s41593-018-0285-z
Lu X, Wang Q, Hu G et al (2009) ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev 23(16):1882–1894. https://doi.org/10.1101/gad.1824809
Masui T, Hosotani R, Tsuji S et al (2001) Expression of METH-1 and METH-2 in pancreatic cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 7(11):3437–3443
Hirano T, Hirose K, Sakurai K et al (2011) Inhibition of tumor growth by antibody to ADAMTS1 in mouse xenografts of breast cancer. Anticancer Res 31(11):3839–3842
Eissa MAL, Lerner L, Abdelfatah E et al (2019) Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin Epigenetics 11(1):59. https://doi.org/10.1186/s13148-019-0650-0
Ricciardelli C, Frewin KM, Tan Ide A et al (2011) The ADAMTS1 protease gene is required for mammary tumor growth and metastasis. Am J Pathol 179(6):3075–3085. https://doi.org/10.1016/j.ajpath.2011.08.021
Bonuccelli G, Casimiro MC, Sotgia F et al (2009) Caveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. Am J Pathol 174(5):1650–1662. https://doi.org/10.2353/ajpath.2009.080648
Du H, Shih CH, Wosczyna MN et al (2017) Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun 8(1):669. https://doi.org/10.1038/s41467-017-00522-7
Peris-Torres C, Plaza-Calonge MDC, López-Domínguez R et al (2020) Extracellular protease ADAMTS1 is required at early stages of human uveal melanoma development by inducing stemness and endothelial-like features on tumor cells. Cancers. https://doi.org/10.3390/cancers12040801
Kore RA, Edmondson JL, Jenkins SV et al (2018) Hypoxia-derived exosomes induce putative altered pathways in biosynthesis and ion regulatory channels in glioblastoma cells. Biochem Biophys Rep 14:104–113. https://doi.org/10.1016/j.bbrep.2018.03.008
Martino-Echarri E, Fernández-Rodríguez R, Bech-Serra JJ et al (2014) Relevance of IGFBP2 proteolysis in glioma and contribution of the extracellular protease ADAMTS1. Oncotarget 5(12):4295–4304. https://doi.org/10.18632/oncotarget.2009
Serrano-Garrido O, Peris-Torres C, Redondo-García S et al (2020) ADAMTS1 supports endothelial plasticity of glioblastoma cells with relevance for glioma progression. Biomolecules 11(1):44. https://doi.org/10.3390/biom11010044
Funding
This work was supported by Tianjin Natural Science Foundation (grant number: 21JCQNJC01610), National Natural Science Foundation of China (grant number: 82003134), Natural Science Foundation of Beijing Municipality (grant number: 7222053), China Postdoctoral Science Foundation (grant number: 2020M670382, 2019T120115), Beijing Financial Support for Postdoctoral Research (grant number: ZZ2019-08) and Tianjin Key Medical Discipline (Specialty) Construction Project (grant number: TJYXZDXK-009A).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material, preparation, data collection and analysis were performed by SW, JZ, KW, YZ, DL. The first draft of the manuscript was written by SW and JZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Wang, S., Zhang, J., Wang, K. et al. ADAMTS1 as potential prognostic biomarker promotes malignant invasion of glioma. Int J Clin Oncol 28, 52–68 (2023). https://doi.org/10.1007/s10147-022-02268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02268-9