Abstract
Background
Human papillomavirus vaccination is not widespread in Japan, and the low screening rates result in many cases of locally advanced cervical cancer. We investigated the prognostic significance of sarcopenia in patients with cervical cancer to guide healthcare policies to improve treatment outcomes.
Methods
This retrospective study included 83 patients with cervical cancer without distant metastasis who underwent primary concurrent chemoradiotherapy between 2013 and 2018. We analyzed the indicators of physical condition and muscle quantity using the SYNAPSE VINCENT software. Muscle mass and the relationship between treatment toxicity and prognosis were evaluated.
Results
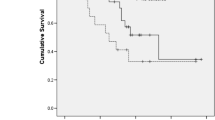
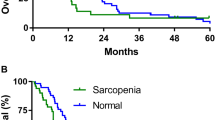
The patients’ median age was 60 (range 33‒80) years. Cancer stage distribution was as follows: cT2b or higher, 84.3%; N1, 65.1%; and MA, 27.7%. The overall sarcopenia (skeletal muscle index [SMI] < 38.5) rate was 30.1%, and the rate was 33.9 and 22.2% in patients aged < 64 and ≥ 65 years, respectively. No correlation was observed between clinical stage and musculoskeletal indices. Treatment resulted in decreased body weight and SMI; after treatment, the sarcopenia rate increased to 37.3%. A higher intramuscular adipose tissue content (IMAC) reduced the number of chemotherapy cycles needed. Treatment-associated SMI decreases of ≥ 7% indicated poor prognosis, with significant differences in progression-free survival and overall survival (p = 0.013 and p = 0.012, respectively). Patients who were very lean (body mass index < 18.5 kg/m2) before treatment had a poor prognosis (p = 0.016 and p < 0.001).
Conclusions
Our findings emphasize the importance of assessing original nutritional status and maintaining muscle mass and quality during the treatment of patients with cervical cancer.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Castellsagué X, Muñoz N (2003) Chapter 3: Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 31:20–28
Hanley SJ, Yoshioka E, Ito Y et al (2015) HPV vaccination crisis in Japan. Lancet 385:2571
Saito T, Katabuchi H (2016) Annual report of the committee on gynecologic oncology, Japan society of obstetrics and gynecology: patient annual report for 2013 and treatment annual report for 2008. J Obstet Gynaecol Res 42:1069–1079
Quinten C, Coens C, Ghislain I et al (2015) The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European organisation for research and treatment of cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer 51:2808–2819
Rosenberg I (1989) Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 50:1231–1233
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Pamoukdjian F, Bouillet T, Lévy V et al (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37:1101–1113
Shachar SS, Deal AM, Weinberg M et al (2017) Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res 23:658–665
Zhou X, Wang JL, Lu J et al (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142:531–543
Gadducci A, Cosio S (2021) The prognostic relevance of computed tomography-assessed skeletal muscle index and skeletal muscle radiation attenuation in patients with gynecological cancer. Anticancer Res 41:9–20
Lee J, Lin JB, Wu MH et al (2020) Muscle loss after chemoradiotherapy as a biomarker of distant failures in locally advanced cervical cancer. Cancers (Basel) 12:595
Allanson ER, Peng Y, Choi A et al (2020) A systematic review and meta-analysis of sarcopenia as a prognostic factor in gynecological malignancy. Int J Gynecol Cancer 30:1791–1797
Anjanappa M, Corden M, Green A et al (2020) Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol 16:50–57
Kiyotoki T, Nakamura K, Haraga J et al (2018) Sarcopenia is an important prognostic factor in patients with cervical cancer undergoing concurrent chemoradiotherapy. Int J Gynecol Cancer 28:168–175
Matsuoka H, Nakamura K, Matsubara Y et al (2019) Sarcopenia is not a prognostic factor of outcome in patients with cervical cancer undergoing concurrent chemoradiotherapy or radiotherapy. Anticancer Res 39:933–939
Hamaguchi Y, Kaido T, Okumura S et al (2017) Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 101:565–574
Yoshimura T, Suzuki H, Takayama T et al (2020) Impact of preoperative low prognostic nutritional index and high intramuscular adipose tissue content on outcomes of patients with oral squamous cell carcinoma. Cancers 12:3167
Ojima Y, Harano M, Sumitani D et al (2019) Impact of preoperative skeletal muscle mass and quality on the survival of elderly patients after curative resection of colorectal cancer. J Anus Rectum Colon 3:143–151
Common terminology criteria for adverse events (2020) Available via https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed Mar 2021
Sánchez M, Castro-Eguiluz D, Luvián-Morales J et al (2019) Deterioration of nutritional status of patients with locally advanced cervical cancer during treatment with concomitant chemoradiotherapy. J Hum Nutr Diet 32:480–491
Gillen J, Mills KA, Dvorak J et al (2019) Imaging biomarkers of adiposity and sarcopenia as potential predictors for overall survival among patients with endometrial cancer treated with bevacizumab. Gynecol Oncol Rep 30:100502
Poorolajal J, Jenabi E (2016) The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev 25:232–238
Kyrgiou M, Kalliala I, Markozannes G et al (2017) Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 356:j477
Shepshelovich D, Xu W, Lu L et al (2019) Body mass index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the International lung cancer consortium. J Thorac Oncol 14:1594–1607
Minami S, Ihara S, Tanaka T et al (2020) Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World J Oncol 11:9–22
Anker SD, Morley JE, von Haehling S (2016) Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle 7:512–514
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Chen LK, Woo J, Assantachai P et al (2020) Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21:300-307.e2
Lee J, Chang CL, Lin JB et al (2018) Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin Cancer Res 24:5028–5036
Malmstrom TK, Morley JE (2013) SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 14:531–532
Acknowledgements
We wish to appreciate M Yuasa for providing support in the body composition analysis of the computed tomography images of the patients examined in this study.
Funding
This research received no specific funding from any agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AA and MY. The first draft of the manuscript was written by AA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Abe, A., Yuasa, M., Imai, Y. et al. Extreme leanness, lower skeletal muscle quality, and loss of muscle mass during treatment are predictors of poor prognosis in cervical cancer treated with concurrent chemoradiation therapy. Int J Clin Oncol 27, 983–991 (2022). https://doi.org/10.1007/s10147-022-02140-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02140-w