Abstract
Background
We here applied cancer personalized profiling by deep sequencing (CAPP-seq) to analysis of circulating tumor DNA (ctDNA) to identify resistance mechanisms in osimertinib-treated patients with EGFR T790M–positive non–small cell lung cancer (NSCLC).
Methods
The study included patients with EGFR activating mutation–positive advanced NSCLC who were positive for T790M in tumor tissue or plasma after previous treatment with an EGFR tyrosine kinase inhibitor, who received osimertinib at Kindai University Hospital between August 2014 and September 2017, and for whom plasma collected after progression on osimertinib was available. Clinical data were extracted from medical records. Patients with innate resistance to osimertinib were defined as those whose best response was progressive disease or stable disease for < 6 months, whereas patients with a complete or partial response or stable disease for > 6 months were considered as having acquired resistance.
Results
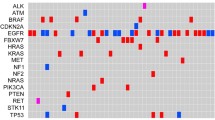
We performed CAPP-seq for 20 patients at progression on osimertinib. Distinct patterns of genomic alterations were apparent in patients with innate versus acquired resistance. Mutations in PIK3CA, KRAS, or BRAF and copy number gain for EGFR, ERBB2, or MET were more common in patients with innate resistance than in those with acquired resistance. In addition, one patient who underwent a repeat biopsy was found to harbor the C797S mutation of EGFR after disease progression during osimertinib rechallenge, with this mutation not having been detected at the time of initial progression on osimertinib.
Conclusions
CAPP-seq analysis of ctDNA was able to identify potentially targetable genetic alterations in patients with osimertinib resistance.





Similar content being viewed by others
References
Mok TS, Wu YL, Ahn MJ et al (2017) Osimertinib or Platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640. https://doi.org/10.1056/NEJMoa1612674
Yang JC, Ahn MJ, Kim DW et al (2017) Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35(12):1288–1296. https://doi.org/10.1200/JCO.2016.70.3223
Oxnard GR, Hu Y, Mileham KF et al (2018) Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 4(11):1527–1534. https://doi.org/10.1001/jamaoncol.2018.2969
Planchard D, Loriot Y, Andre F et al (2015) EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 26(10):2073–2078. https://doi.org/10.1093/annonc/mdv319
Ou SI, Nagasaka M, Zhu VW (2018) Liquid biopsy to identify actionable genomic alterations. Am Soc Clin Oncol Educ Book 38:978–997. https://doi.org/10.1200/EDBK_199765
Yang Z, Yang N, Ou Q et al (2018) Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res 24(13):3097–3107. https://doi.org/10.1158/1078-0432.CCR-17-2310
Le X, Puri S, Negrao MV et al (2018) Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res 24(24):6195–6203. https://doi.org/10.1158/1078-0432.CCR-18-1542
Piotrowska Z, Isozaki H, Lennerz JK et al (2018) Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov 8(12):1529–1539. https://doi.org/10.1158/2159-8290.CD-18-1022
Thress KS, Paweletz CP, Felip E et al (2015) Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21(6):560–562. https://doi.org/10.1038/nm.3854
Lin CC, Shih JY, Yu CJ et al (2018) Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med 6(2):107–116. https://doi.org/10.1016/S2213-2600(17)30480-0
Newman AM, Bratman SV, To J et al (2014) An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 20(5):548–554. https://doi.org/10.1038/nm.3519
Newman AM, Lovejoy AF, Klass DM et al (2016) Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 34(5):547–555. https://doi.org/10.1038/nbt.3520
Scherer F, Kurtz DM, Newman AM et al (2016) Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med 8(364):364ra155. https://doi.org/10.1126/scitranslmed.aai8545
Jeong Y, Hoang NT, Lovejoy A et al (2017) Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov 7(1):86–101. https://doi.org/10.1158/2159-8290.CD-16-0127
Dudley JC, Schroers-Martin J, Lazzareschi DV et al (2019) Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov 9(4):500–509. https://doi.org/10.1158/2159-8290.CD-18-0825
Chaudhuri AA, Chabon JJ, Lovejoy AF et al (2017) Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 7(12):1394–1403. https://doi.org/10.1158/2159-8290.CD-17-0716
Chabon JJ, Simmons AD, Lovejoy AF et al (2016) Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 7:11815. https://doi.org/10.1038/ncomms11815
Przybyl J, Chabon JJ, Spans L et al (2018) Combination approach for detecting different types of alterations in circulating tumor DNA in leiomyosarcoma. Clin Cancer Res 24(11):2688–2699. https://doi.org/10.1158/1078-0432.CCR-17-3704
Otsubo K, Sakai K, Takeshita M et al (2019) Genetic profiling of non-small cell lung cancer at development of resistance to first- or second-generation EGFR-TKIs by CAPP-seq analysis of circulating tumor DNA. Oncologist 24(8):1022–1026. https://doi.org/10.1634/theoncologist.2019-0101
Jackman D, Pao W, Riely GJ et al (2010) Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 28(2):357–360. https://doi.org/10.1200/JCO.2009.24.7049
Talevich E, Shain AH, Botton T et al (2016) CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol 12(4):e1004873. https://doi.org/10.1371/journal.pcbi.1004873
Robinson JT, Thorvaldsdottir H, Winckler W et al (2011) Integrative genomics viewer. Nat Biotechnol 29(1):24–26. https://doi.org/10.1038/nbt.1754
Varella-Garcia M (2006) Stratification of non-small cell lung cancer patients for therapy with epidermal growth factor receptor inhibitors: the EGFR fluorescence in situ hybridization assay. Diagn Pathol 1:19. https://doi.org/10.1186/1746-1596-1-19
Go H, Jeon YK, Park HJ et al (2010) High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol 5(3):305–313. https://doi.org/10.1097/JTO.0b013e3181ce3d1d
Ishii H, Azuma K, Sakai K et al (2015) Digital PCR analysis of plasma cell-free DNA for non-invasive detection of drug resistance mechanisms in EGFR mutant NSCLC: correlation with paired tumor samples. Oncotarget 6(31):30850–30858. https://doi.org/10.18632/oncotarget.5068
Takegawa N, Yonesaka K, Sakai K et al (2016) HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget 7(3):3453–3460. https://doi.org/10.18632/oncotarget.6498
Offin M, Chan JM, Tenet M et al (2019) Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol 14(10):1784–1793. https://doi.org/10.1016/j.jtho.2019.06.002
Sequist LV, Waltman BA, Dias-Santagata D et al (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3(75):75ra26. https://doi.org/10.1126/scitranslmed.3002003
Kato S, Okamura R, Mareboina M et al (2019) Revisiting epidermal growth factor receptor (EGFR) amplification as a target for anti-EGFR therapy: analysis of cell-free circulating tumor DNA in patients with advanced malignancies. JCO Precis Oncol. https://doi.org/10.1200/PO.18.00180
Lam SN, Zhou YC, Chan YM et al (2020) Comparison of target enrichment platforms for circulating tumor DNA detection. Sci Rep 10(1):4124. https://doi.org/10.1038/s41598-020-60375-x
Zhu YJ, Zhang HB, Liu YH et al (2017) Quantitative cell-free circulating EGFR mutation concentration is correlated with tumor burden in advanced NSCLC patients. Lung Cancer 109:124–127. https://doi.org/10.1016/j.lungcan.2017.05.005
Yanagita M, Redig AJ, Paweletz CP et al (2016) A prospective evaluation of circulating tumor cells and cell-free DNA in EGFR-mutant non-small cell lung cancer patients treated with erlotinib on a phase II trial. Clin Cancer Res 22(24):6010–6020. https://doi.org/10.1158/1078-0432.CCR-16-0909
Corcoran RB, Chabner BA (2018) Application of cell-free DNA analysis to cancer treatment. N Engl J Med 379(18):1754–1765. https://doi.org/10.1056/NEJMra1706174
Keller L, Belloum Y, Wikman H et al (2021) Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer 124(2):345–358. https://doi.org/10.1038/s41416-020-01047-5
Nukaga S, Yasuda H, Tsuchihara K et al (2017) Amplification of EGFR wild-type alleles in non-small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitors. Cancer Res 77(8):2078–2089. https://doi.org/10.1158/0008-5472.CAN-16-2359
Wu YL, Zhang L, Kim DW et al (2018) Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol 36(31):3101–3109. https://doi.org/10.1200/JCO.2018.77.7326
Ercan D, Choi HG, Yun CH et al (2015) EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res 21(17):3913–3923. https://doi.org/10.1158/1078-0432.CCR-14-2789
Niederst MJ, Hu H, Mulvey HE et al (2015) The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 21(17):3924–3933. https://doi.org/10.1158/1078-0432.CCR-15-0560
Hata A, Katakami N, Yoshioka H et al (2015) Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J Thorac Oncol 10(11):1553–1559. https://doi.org/10.1097/JTO.0000000000000647
Ichihara E, Hotta K, Kubo T et al (2018) Clinical significance of repeat rebiopsy in detecting the EGFR T790M secondary mutation in patients with non-small cell lung cancer. Oncotarget 9(50):29525–29531. https://doi.org/10.18632/oncotarget.25705
Soria JC, Ohe Y, Vansteenkiste J et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125. https://doi.org/10.1056/NEJMoa1713137
Ramalingam SS, Vansteenkiste J, Planchard D et al (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382(1):41–50. https://doi.org/10.1056/NEJMoa1913662
Acknowledgements
We thank Tomoki Yamanaka of Roche Diagnostics as well as Haruka Sakamoto, Yume Shinkai, Michiko Kitano, and Mami Kitano of Kindai University for technical support.
Funding
This work was supported by Nippon Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Kato has received lecture fees from Bristol-Myers Squibb Co. Ltd.; and honoraria from Eli Lilly Japan K.K. H. Hayashi has received honoraria from AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Merck Biopharma Co. Ltd., and Taiho Pharmaceutical Co. Ltd.; and research funding from AbbVie Inc., AC MEDICAL Inc., Astellas Pharma Inc., AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., EPS Associates Co. Ltd., GlaxoSmithKline K.K., Japan Clinical Research Operations Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., PAREXEL International Corp., Pfizer Japan Inc., PPD-SNBL K.K., Quintiles Transnational Japan K.K., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Yakult Honsha Co. Ltd. K. Sakai has received lecture fees from AstraZeneca K.K., Bio-Rad Laboratories, Inc., Chugai Pharmaceutical Co. Ltd., and Roche Diagnostics K.K., SRL, Inc. S. Suzuki has declared no conflicts of interest.K. Haratani has received honoraria from AstraZeneca K.K.; lecture fees from AS ONE Corporation, AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD K.K., and Pfizer Japan Inc.; and research funding from AstraZeneca K.K. T. Takahama has received honoraria from AstraZeneca K.K., Roche Diagnostics K.K., and Nippon Boehringer Ingelheim Co. Ltd. J. Tanizaki has received honoraria from AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd, Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., and Taiho Pharmaceutical Co. Ltd. Y. Nonagase has received honoraria from Taiho Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd. K. Tanaka has received honoraria from AstraZeneca KK, Bristol-Myers Squibb, Eisai Co. Ltd., Merck Biopharma Co., and Ono Pharmaceutical Co. Ltd. T. Yoshida has received lecture fees from Bristol-Myers Squibb Co. Ltd., Daiichi Sankyo Co. Ltd., Hisamitsu Pharmaceutical Co. Inc., MundiPharma K.K., and Shionogi & Co. Ltd. M. Takeda has received honoraria from Chugai Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., and Ono Pharmaceutical Co. Ltd. K. Yonesaka has received research funding from Daiichi Sankyo Co. Ltd.; and honoraria from Nippon Boehringer Ingelheim Co. Ltd. H. Kaneda has received lecture fees from AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Nippon Kayaku Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Taiho Pharmaceutical Co. Ltd.; and research funding from Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Novartis Pharma K.K., and Ono Pharmaceutical Co. Ltd. K. Nishio has received honoraria from AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., Guardant Health Inc., Kobayashi Pharmaceutical Co. Ltd., Life Technologies Japan Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Pfizer Japan Inc., Roche Diagnostics K.K., Sanofi K.K., Solasia Pharma K.K., SymBio Pharmaceuticals Ltd., Takeda Pharmaceutical Co. Ltd., and Yakult Honsha Co. Ltd.; and research funding from Astellas Pharma Inc., Eli Lilly Japan K.K., Ignyta Inc., Korea Otsuka Co. Ltd., Life Technologies Japan Ltd., and Nippon Boehringer Ingelheim Co. Ltd. K. Nakagawa has received honoraria from Astellas Pharma Inc., AstraZeneca K.K., AYUMI Pharmaceutical Co., Bristol-Myers Squibb Co. Ltd., CareNet Inc., Chugai Pharmaceutical Co. Ltd., Clinical Trial Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly Japan K.K., Hisamitsu Pharmaceutical Co. Inc., KYORIN Pharmaceutical Co. Ltd., Medical Review Co. Ltd., MEDICUS SHUPPAN Publishers Co. Ltd., MSD K.K., NANZANDO Co. Ltd., Nichi-Iko Pharmaceutical Co. Ltd., Nikkei Business Publications Inc., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Reno. Medical K.K., and SymBio Pharmaceuticals Ltd., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Thermo Fisher Scientific K.K., YODOSHA Co. Ltd, and YOMIURI TELECASTING Co.; and research funding from A2 Healthcare Corp., AbbVie Inc., Astellas Pharma Inc., Bayer Yakuhin Ltd., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., CMIC Shift Zero K.K., Covance Inc., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Eli Lilly Japan K.K., EP-CRSU Co. Ltd., EPS Co., EPS international Co. Ltd., GlaxoSmithKline K.K., GRITSTONE ONCOLOGY Inc., ICON Japan K.K., inVentiv Health Japan, IQVIA Services JAPAN K.K., Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Linical Co. Ltd., Merch Serono Co. Ltd., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., PAREXEL International Corp., Pfizer Japan Inc., Quintiles Inc., SymBio Pharmaceuticals Ltd., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Yakult Honsha Co. Ltd.; and consulting or advisory fees from Astellas Pharma Inc., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kato, R., Hayashi, H., Sakai, K. et al. CAPP-seq analysis of circulating tumor DNA from patients with EGFR T790M–positive lung cancer after osimertinib. Int J Clin Oncol 26, 1628–1639 (2021). https://doi.org/10.1007/s10147-021-01947-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01947-3