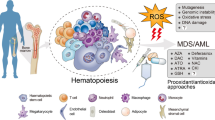
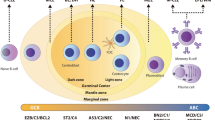
Abstract
Myelodysplastic syndromes (MDS) are a heterogeneous group of myeloid malignancies characterized by peripheral blood cytopenia and dishematopoiesis and frequently progress to acute myeloid leukemia. Genetic defects play a major role in pathogenesis of MDS, including cytogenetic abnormalities, gene mutations, and abnormal gene expression. Chromosomal abnormalities have been detected in approximately 50–60% of MDS patients, including the deletions of chromosome 5q and 7q, trisomy 8, and complex karyotypes. Newer genomic technologies, such as single-nucleotide polymorphism array and next-generation sequencing, revealed the heterozygous deletions resulting in haploinsufficient gene expression (e.g., CSNK1A1, DDX41 on chromosome 5, CUX1, LUC7L2, EZH2 on chromosome 7) involved in the pathogenesis of MDS. In addition, recurrent somatic mutations in more than 50 genes have been identified in 80–90% of MDS. The most recurrent genetic mutations are involved in the RNA splicing (e.g., SF3B1, SRSF2, U2AF1, ZRSR2, LUC7L2, DDX41) and epigenetic modifications, such as histone modification (e.g., ASXL1, EZH2) and DNA methylation (e.g., TET2, DNMT3A, IDH1/IDH2). TP53 mutation is associated with aggressive disease and frequently coincides with deletion of chromosome 5q. This review summarizes the recent progress in molecular pathogenesis of MDS. A better understanding of the specific subgroups of MDS patients will also aid in the development of new therapeutic approach for MDS.


Similar content being viewed by others
References
Pellagatti A, Boultwood J (2015) The molecular pathogenesis of the myelodysplastic syndromes. Eur J Haematol 95:3–15
Gondek LP, Tiu R, O'Keefe CL et al (2008) Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood 111:1534–1542
Brunning R, Orazi A, Germing U et al (2008) Myelodysplastic syndromes/neoplasms, overview. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, Lyon, pp 88–93
Yoshida K, Sanada M, Shiraishi Y et al (2011) Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478:64–69
Greenberg PL, Tuechler H, Schanz J et al (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454–2465
Sole F, Espinet B, Sanz GF et al (2000) Incidence, characterization and prognostic significance of chromosomal abnormalities in 640 patients with primary myelodysplastic syndromes. Grupo Cooperativo Espanol de Citogenetica Hematologica. Br J Haematol 108:346–356
Boultwood J, Fidler C, Strickson AJ et al (2002) Narrowing and genomic annotation of the commonly deleted region of the 5q- syndrome. Blood 99:4638–4641
Jerez A, Gondek LP, Jankowska AM et al (2012) Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J Clin Oncol 30:1343–1349
Patnaik MM, Lasho TL, Finke CM et al (2010) WHO-defined 'myelodysplastic syndrome with isolated del(5q)' in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia 24:1283–1289
Nimer SD (2006) Clinical management of myelodysplastic syndromes with interstitial deletion of chromosome 5q. J Clin Oncol 24:2576–3282
Grimwade D, Walker H, Oliver F et al (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The medical research council adult and children's leukaemia working parties. Blood 92:2322–2333
Byrd JC, Mrozek K, Dodge RK et al (2002) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100:4325–4336
Lai F, Godley LA, Joslin J et al (2001) Transcript map and comparative analysis of the 1.5-Mb commonly deleted segment of human 5q31 in malignant myeloid diseases with a del(5q). Genomics 71:235–245
Ebert BL, Pretz J, Bosco J et al (2008) Identification of RPS14 as a 5q-syndrome gene by RNA interference screen. Nature 451:335–339
Starczynowski DT, Kuchenbauer F, Argiropoulos B et al (2010) Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat Med 16:49–58
Schneider RK, Adema V, Heckl D et al (2014) Role of casein kinase 1A1 in the biology and targeted therapy of del(5q) MDS. Cancer Cell 26:509–520
Krönke J, Fink EC, Hollenbach PW et al (2015) Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 523:183–188
Soncini C, Berdo I, Draetta G (2001) Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene 20:3869–3879
Hosono N, Makishima H, Mahfouz R et al (2017) Recurrent genetic defects on chromosome 5q in myeloid neoplasms. Oncotarget 8:6483–6495
Polprasert C, Schulze I, Sekeres MA et al (2015) Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell 27:658–670
Maciejewski JP, Padgett RA, Brown AL et al (2017) DDX41-related myeloid neoplasia. Semin Hematol 54:94–97
Jädersten M, Saft L, Pellagatti A et al (2009) Clonal heterogeneity in the 5q-syndrome: p53 expressing progenitors prevail during lenalidomide treatment and expand at disease progression. Haematologica 94:1762–1766
Möllgård L, Saft L, Treppendahl MB et al (2011) Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica 96:963–971
Kantarjian H, O'Brien S, Ravandi F et al (2009) The heterogeneous prognosis of patients with myelodysplastic syndrome and chromosome 5 abnormalities. Cancer 115:5202–5209
Okamoto K, Li HY, Jensen MR et al (2002) Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol Cell 9:761–771
Bernasconi P, Klersy C, Boni M et al (2005) Incidence and prognostic significance of karyotype abnormalities in de novo primary myelodysplastic syndromes: a study on 331 patients from a single institution. Leukemia 19:1424–1431
Haase D, Germing U, Schanz J et al (2007) New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 110:4385–4395
Schanz J, Tuchler H, Sole F et al (2012) New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 30:820–829
Jerez A, Sugimoto Y, Makishima H et al (2015) Loss of heterozygosity in 7q myeloid disorders: clinical associations and genomic pathogenesis. Blood 119:6109–6117
Hosono N, Makishima H, Jerez A et al (2014) Recurrent genetic defects on chromosome 7q in myeloid neoplasms. Leukemia 28:1348–1351
Wong CC, Martincorena I, Rust AG et al (2014) Inactivating CUX1 mutations promote tumorigenesis. Nat Genet 46:33–38
McNerney ME, Brown CD, Wang X et al (2013) CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood 121:975–983
Papaemmanuil E, Gerstung M, Malcovati L et al (2013) Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122:3616–3627
Ramdzan ZM, Pal R, Kaur S (2015) The function of CUX1 in oxidative DNA damage repair is needed to prevent premature senescence of mouse embryo fibroblasts. Oncotarget 6:3613–3626
Ramdzan ZM, Nepveu A (2014) CUX1, a haploinsufficient tumour suppressor gene overexpressed in advanced cancers. Nat Rev Cancer 14:673–682
Puig O, Bragado-Nilsson E, Koski T et al (2007) The U1 snRNP-associated factor Luc7p affects 5' splice site selection in yeast and human. Nucleic Acids Res 35:5874–5885
Hershberger CE, Hosono N, Singh J et al (2016) (2016) The role of LUC7L2 in splicing and MDS. Blood 128:5504
Simon JA, Kingston RE (2009) Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 10:697–708
Ernst T, Chase AJ, Score J et al (2010) Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 42:722–726
Nikoloski G, Langemeijer SM, Kuiper RP et al (2010) Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet 42:665–667
Makishima H, Jankowska AM, Tiu RV et al (2010) Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia 24:1799–1804
Nagamachi A, Matsui H, Asou H et al (2013) Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell 24:305–317
Inaba T, Honda H, Matsui H et al (2018) The enigma of monosomy 7. Blood 131:2891–2898
Paulsson K, Säll T, Fioretos T et al (2001) The incidence of trisomy 8 as a sole chromosomal aberration in myeloid malignancies varies in relation to gender, age, prior iatrogenic genotoxic exposure, and morphology. Cancer Genet Cytogenet 130:160–165
Nilsson L, Åstrand-Grundström I, Anderson K et al (2002) Involvement and functional impairment of the CD34+ CD38− Thy-1+ hematopoietic stem cell pool in myelodysplastic syndromes with trisomy 8. Blood 100:259–267
de Souza Fernandez T, Ornellas MH, de Carvalho LO et al (2000) Chromosomal alterations associated with evolution from myelodysplastic syndrome to acute myeloid leukemia. Leuk Res 24:839–848
Sloand EM, Pfannes L, Chen G et al (2007) CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood 109:2399–2405
Makishima H, Yoshizato T, Yoshida K et al (2017) Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 49:204–212
Wahl MC, Will CL, Lührmann R (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136:701–718
Graubert TA, Shen D, Ding L et al (2011) Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet 44:53–57
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BL (2011) Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 364:2496–2506
Score J, Hidalgo-Curtis C, Jones AV et al (2012) Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Blood 119:1208–1213
Ueda T, Sanada M, Matsui H et al (2012) EED mutants impair polycomb repressive complex 2 in myelodysplastic syndrome and related neoplasms. Leukemia 26:2557–2560
Khan SN, Jankowska AM, Mahfouz R et al (2013) Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies. Leukemia 27:1301–1309
Shi J, Wang E, Zuber J et al (2013) The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;NrasG12D acute myeloid leukemia. Oncogene 32:930–938
Margueron R, Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469:343–349
Boultwood J, Perry J, Pellagatti A et al (2010) Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia 24:1062–1065
Yang H, Kurtenbach S, Guo Y et al (2018) Gain of function of ASXL1 truncating protein in the pathogenesis of myeloid malignancies. Blood 131:328–341
Walter MJ, Ding L, Shen D et al (2011) Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia 25:1153–1158
Genovese G, Kahler AK, Handsaker RE et al (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371:2477–2487
Delhommeau F, Dupont S, Della Valle V et al (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 2009:2289–2301
Ko M, Huang Y, Jankowska AM et al (2010) Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468:839–843
Yamazaki J, Taby R, Vasanthakumar A et al (2012) Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics 7:201–207
Lin CC, Hou HA, Chou WC et al (2014) IDH mutations are closely associated with mutations of DNMT3A, ASXL1 and SRSF2 in patients with myelodysplastic syndromes and are stable during disease evolution. Am J Hematol 89:137–144
DiNardo CD, Jabbour E, Ravandi F et al (2016) IDH1 and IDH2 mutations in myelodysplastic syndromes and role in disease progression. Leukemia 30:980–984
Haferlach T, Nagata Y, Grossmann V et al (2014) Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 28:241–247
Mardis ER, Ding L, Dooling DJ et al (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361:1058–1066
Prensner JR, Chinnaiyan AM (2011) Metabolism unhinged: IDH mutations in cancer. Nat Med 17:291–293
Bejar R, Stevenson KE, Caughey B, Lindsley RC et al (2014) Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stemcell transplantation. J Clin Oncol 32:2691–2698
Montalban-Bravo G, Takahashi K, Patel K, Wang F et al (2018) Impact of the number of mutations in survival and response outcomes to hypomethylating agents in patients with myelodysplastic syndromes or myelodysplastic/ myeloproliferative neoplasms. Oncotarget 9:9714–9727
Jadersten M, Saft L, Smith A, Kulasekararaj A et al (2011) TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 29:1971–1979
Scharenberg C, Giai V, Pellagatti A, Saft L et al (2017) Progression in patients with low- and intermediate-1-risk del(5q) myelodysplastic syndromes is predicted by a limited subset of mutations. Haematologica 102:498–508
Lindsley RC, Mar BG, Mazzola E, Grauman PV et al (2015) Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125:1367–1376
Wong TN, Miller CA, Jotte MRM, Bagegni N et al (2018) Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun 9:455
Welch JS, Petti AA, Miller CA et al (2016) TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 375:2023–2036
Welch JS, Petti AA, Ley TJ (2017) Decitabine in TP53-mutated AML. N Engl J Med 376:797–798
Bykov VJ, Issaeva N, Shilov A et al (2002) Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8:282–288
Kwok B, Hall JM, Witte JS et al (2015) MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 126:2355–2361
Steensma DP, Bejar R, Jaiswal S et al (2015) Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126:9–16
Acknowledgements
First and foremost, the author would like to thank Professor Jaroslaw P. Maciejewski, Department of Translational Hematology and Oncology Research, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH and Professor Hideki Makishima, Department of Pathology and Tumor Biology, Graduate School of Medicine, Kyoto University. This work was supported by JSPS KAKENHI Grant Number JP 16K09844 and The Naito Foundation Naito Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard tojurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hosono, N. Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol 24, 885–892 (2019). https://doi.org/10.1007/s10147-019-01462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01462-6