Abstract
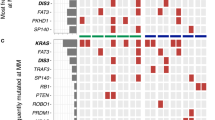
Multiple myeloma (MM), one of the most intractable malignancies, is characterized by the infiltration and growth of plasma cells, the most differentiated cells in the B-cell lineage, in the bone marrow. Despite the introduction of novel therapeutic agents, including proteasome inhibitors and immunomodulatory drugs, the prognosis of patients with MM is still worse than that of most hematological malignancies. A better understanding of the molecular pathogenesis of the disease is essential to achieve any improvement of treatment outcome of MM patients. All MM cases pass through the phase of asymptomatic expansion of clonal plasma cells, referred to as monoclonal gammopathy of undetermined significance (MGUS). It has long been believed that MM evolves linearly from MGUS to terminal phases, such as extramedullary tumors and plasma cell leukemia via the accumulation of novel mutations. However, recent studies using next-generation sequencing have disclosed the complex genomic architecture of the disease. At each step of progression, the acquisition of novel mutations is accompanied by subclonal evolution from reservoir clones with branching patterns. Each subclone may carry novel mutations and distinct phenotypes, including drug sensitivity. In addition, minor clones already exist at the MGUS stage, which could expand later in the clinical course, resulting in relapse and/or leukemic conversion. The ultimate goal of treatment is to eradicate all clones, including subclonal populations with distinct biological characteristics. This goal could be achieved by further improving treatment strategies that reflect the genomic landscape of the disease.







Similar content being viewed by others
References
Palumbo A, Anderson K (2011) Multiple myelomas. N Engl J Med 364:1046–1060
Mitsiades CS, Davies FE, Laubach JP et al (2011) Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J Clin Oncol 29:1916–1923
Corre J, Munshi N, Avet-Loiseau H (2015) Genetics of multiple myeloma: another heterogeneity level? Blood 125:1870–1876
Gay F, Larocca A, Wijermans P et al (2011) Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood 117:3025–3031
Bringhen S, Mateos MV, Zweegman S et al (2013) Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 98:980–987
Sonneveld P, Goldschmidt H, Rosiñol L et al (2013) Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol 31:3279–3287
Kumar SK, Dispenzieri A, Lacy MQ et al (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28:1122–1128
Weiss BM, Abadie J, Verma P et al (2009) A monoclonal precedes multiple myeloma in most patients. Blood 113:5418–5422
Agarwal A, Ghobrial IM (2013) Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: a review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin Cancer Res 19:985–994
Hideshima T, Mitsiades C, Tonon G et al (2007) Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 7:585–598
Meads MB, Hazlehurst LA, Dalton WS (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14:2519–2526
Noborio-Hatano K, Kikuchi J, Takatoku M et al (2009) Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene 28:231–242
Kikuchi J, Koyama D, Mukai HY et al (2014) Suitable drug combination with bortezomib for multiple myeloma under stroma-free conditions and in contact with fibronectin or bone marrow stromal cells. Int J Hematol 99:726–736
Nutt SL, Taubenheim N, Hasbold J et al (2011) The genetic network controlling plasma cell differentiation. Semin Immunol 23:341–349
Pieper K, Grimbacher B, Eibel H (2013) B-cell biology and development. J Allergy Clin Immunol 131:959–971
Walker BA, Wardell CP, Johnson DC et al (2013) Characterization of IGH locus breakpoints in multiple myeloma indicates a subset of translocations appear to occur in pregerminal center B cells. Blood 121:3413–3419
González D, van der Burg M, García-Sanz R et al (2007) Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood 110:3112–3121
Chesi M, Nardini E, Brents LA et al (1997) Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet 16:260–264
Chesi M, Nardini E, Lim RSC et al (1998) The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood 92:3025–3034
Iida S, Rao PH, Butler M et al (1997) Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet 17:226–230
Chesi M, Bergsagel PL, Brents LA et al (1996) Dysregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines. Blood 88:674–681
Bergsagel PL, Kuehl WM, Zhan F et al (2005) Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood 106:296–303
Lauring J, Abukhdeir AM, Konishi H et al (2008) The multiple myeloma-associated MMSET gene contributes to cellular adhesion, clonogenic growth, and tumorigenicity. Blood 111:856–864
Martinez-Garcia E, Popovic R, Min D-J et al (2011) The MMSET histone methyltransferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood 117:211–220
Pei H, Zhang L, Luo K et al (2011) MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 470:124–128
Hurt EM, Wiestner A, Rosenwald A et al (2004) Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell 5:191–199
Chesi M, Bergsagel PL, Shonukan OO et al (1998) Frequent dysregulation of the c-maf protooncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood 91:4457–4463
Sawyer JR, Waldron JA, Jagannath S et al (1995) Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genet Cytogenet 182:41–49
Laï JL, Zandecki M, Mary JY et al (1995) Improved cytogenetics in multiple myeloma: a study of 151 patients including 117 patients at diagnosis. Blood 85:2490–2497
Calasanz MJ, Cigudosa JC, Odero MD et al (1997) Cytogenetic analysis of 280 patients with multiple myeloma and related disorders: primary breakpoints and clinical correlations. Genes Chromosom Cancer 18:84–93
Smadja NV, Bastard C, Brigaudeau C et al (2001) Hypodiploidy is a major prognostic factor in multiple myeloma. Blood 98:2229–2238
Chapman MA, Lawrence MS, Keats JJ et al (2011) Initial genome sequencing and analysis of multiple myeloma. Nature 471:467–472
Lohr JG, Stojanov P, Carter LS et al (2014) Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell 25:91–101
Alexandrov LB, Nik-Zainal S, Wedge DC et al (2013) Signatures of mutational processes in human cancer. Nature 500:415–421
Bolli N, Avet-Loiseau H, Wedge DC et al (2014) Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun 5:2997. doi:10.1038/ncomms3997
Delmore JE, Issa GC, Lemieux ME et al (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146:904–917
Loven J, Hoke HA, Lin CY et al (2013) Selective inhibition of tumor oncogenesis by disruption of super-enhances. Cell 153:320–334
Chiecchio L, Dagrada GP, Ibrahim AH et al (2009) Timing of acquisition of deletion 13 in plasma cell dyscrasias is dependent on genetic context. Haematologica 94:1708–1713
Annunziata CM, Davis RE, Demchenko Y et al (2007) Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12:115–130
Demchenko YN, Glebov OK, Zingone A et al (2010) Classical and/or alternative NF-κB pathway activation in multiple myeloma. Blood 115:3541–3552
Sawyer JR, Tian E, Heuck CJ et al (2014) Jumping translocations of 1q12 in multiple myeloma: a novel mechanism for deletion of 17p in cytogenetically defined high-risk disease. Blood 123:2504–2512
Affer M, Chesi M, Chen WD et al (2014) Promiscuous MYC locus rearrangements hijack enhancers but mostly super-enhancers to dysregulate MYC expression in multiple myeloma. Leukemia 28:1725–1735
Walker BA, Wargell CP, Brioli A et al (2014) Translocations at 8q24 juxtapose MYC with genes that harbor superenhancers resulting in overexpression and poor prognosis in myeloma patients. Blood Cancer J 4:e191
Avet-Loiseau H, Attal M, Campion L et al (2012) Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol 30:1949–1952
Rajkumar SV, Gupta V, Fonseca R et al (2013) Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia 27:1738–1744
Hebraud B, Leleu X, Lauwers-Cances V et al (2014) Deletion of the 1p32 region is a major independent prognostic factor in young patients with myeloma: the IFM experience on 1195 patients. Leukemia 28:675–679
Walker BA, Wardell CP, Melchor L et al (2014) Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 28:384–390
Egan JB, Shi C-X, Tembe W et al (2012) Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood 120:1060–1066
Keats JJ, Chesi M, Egan JB et al (2012) Clonal competition with alternating dominance in multiple myeloma. Blood 120:1067–1076
Walker BA, Wardell CP, Melchor L et al (2012) Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood 120:1077–1086
Bahlis NJ (2012) Darwinian evolution and tiding clones in multiple myeloma. Blood 120:927–928
Hébraud B, Caillot D, Corre J et al (2013) The translocation t(4;14) can be present only in minor subclones in multiple myeloma. Clin Cancer Res 19:4634–4637
Magrangeas F, Avet-Loiseau H, Gouraud W et al (2013) Minor clone provides a reservoir for relapse in multiple myeloma. Leukemia 27:473–481
Rajkumar SV (2012) Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol 87:79–88
Mikhael JR, Dingli D, Roy V et al (2013) Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 88:360–376
Boyd KD, Ross FM, Chiecchio L et al (2012) A novel prognostic model in myeloma based on co-segregation adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia 26:349–355
Mateos MV, Martinez-Lopez J, Hernandez MT et al (2014) Comparison of sequential vs alternating administration of bortezomib, melphalan, prednisone (VMP) and lenalidomide plus dexamethasone (Rd) in elderly patients with newly diagnosed multiple myeloma (MM): GEM2010MAS65 trial. In: Burns LJ (ed) 56th ASH Annual Meeting. American Society of Hematology, Washington DC, Abstract #178
Mateos MV, Hernandez MT, Giraldo P et al (2013) Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med 369:438–481
Ghobrial IM, Landgren O (2014) How I treat smoldering multiple myeloma. Blood 124:3380–3388
Pineda-Roman M, Bolejack V, Arzoumanian V et al (2007) Complete response in myeloma extends survival without, but not with history of prior monoclonal gammopathy of undetermined significance or smouldering disease. Br J Haematol 136:393–399
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Furukawa, Y., Kikuchi, J. Molecular pathogenesis of multiple myeloma. Int J Clin Oncol 20, 413–422 (2015). https://doi.org/10.1007/s10147-015-0837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0837-0