Abstract
Background
KRAS mutation is widely accepted as a strong, negative predictive marker for anti-epidermal growth factor receptor antibodies, including cetuximab and panitumumab. Previous reports demonstrated approximately 100 % concordance of KRAS status between primary colorectal cancer and liver metastases; however, mismatched KRAS status still occurs.
Methods
KRAS status was evaluated in 105 pairs of formalin-fixed primary colorectal cancer and corresponding liver metastases specimens by direct sequencing. DNA quality of patients displaying mismatched KRAS status between primary tumors and metastases was assessed using a Bioanalyzer.
Results
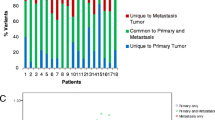
KRAS status was successfully analyzed in 90/105 patients (85.7 %). The concordance rate between primary tumors and metastases was 88.2 % in synchronous metastases (n = 76) and 100 % in metachronous metastases (n = 14). Discordance in KRAS status was observed in nine patients. Independent method validation revealed only five samples showed the same KRAS status between the two methods. DNA quality assessment by a Bioanalyzer revealed that the median length of DNA samples in the peak concentration of the mismatched group was significantly shorter than those in the control group (153.5 vs 276.5 bp, P = 0.0059). In addition, the median value of the percentage of degraded DNA (0–200 bp) in each sample in the mismatched group was significantly higher than the control group (35.5 vs 22 %, P = 0.020). These data suggest that the discordant results for these nine patients (18 samples) were due to low quality DNA, which may obscure polymerase chain reaction analysis, affecting sequencing reliability.
Conclusion
Quality control and assurance of KRAS genotyping is critical, and standardization of the methodology is warranted.

Similar content being viewed by others
References
Douillard JY, Siena S, Cassidy J et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28(31):4697–4705
Lievre A, Bachet JB, Boige V et al (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374–379
Monzon FA, Ogino S, Hammond ME et al (2009) The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med 133(10):1600–1606
Knijn N, Mekenkamp LJ, Klomp M et al (2011) KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 104(6):1020–1026
Pao W, Wang TY, Riely GJ et al (2005) KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2(1):e17
Hoshi K, Takakura H, Mitani Y et al (2007) Rapid detection of epidermal growth factor receptor mutations in lung cancer by the SMart-Amplification Process. Clin Cancer Res 13(17):4974–4983
Mitani Y, Lezhava A, Kawai Y et al (2007) Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat Methods 4(3):257–262
Araki T, Shimizu K, Nakamura K et al (2010) Usefulness of peptide nucleic acid (PNA)-clamp smart amplification process version 2 (SmartAmp2) for clinical diagnosis of KRAS codon 12 mutations in lung adenocarcinoma: comparison of PNA-clamp SmartAmp2 and PCR-related methods. J Mol Diagn 12(1):118–124
Tatsumi K, Mitani Y, Watanabe J et al (2008) Rapid screening assay for KRAS mutations by the modified smart amplification process. J Mol Diagn 10(6):520–526
Taguchi M, Inoue H, Motani-Saitoh H et al (2012) DNA identification of formalin-fixed organs is affected by fixation time and type of fixatives: using the AmpF ℓ STR(R) Identifiler(R) PCR Amplification Kit. Med Sci Law 52(1):12−16
Ferrer I, Armstrong J, Capellari S et al (2007) Effects of formalin fixation, paraffin embedding, and time of storage on DNA preservation in brain tissue: a BrainNet Europe study. Brain Pathol 17(3):297–303
Inoue T, Nabeshima K, Kataoka H et al (1996) Feasibility of archival non-buffered formalin-fixed and paraffin-embedded tissues for PCR amplification: an analysis of resected gastric carcinoma. Pathol Int 46(12):997–1004
Turashvili G, Yang W, McKinney S et al (2012) Nucleic acid quantity and quality from paraffin blocks: defining optimal fixation, processing and DNA/RNA extraction techniques. Exp Mol Pathol 92(1):33–43
Van Cutsem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417
Bando H, Tsuchihara K, Yoshino T et al (2011) Biased discordance of KRAS mutation detection in archived colorectal cancer specimens between the ARMS-Scorpion method and direct sequencing. Jpn J Clin Oncol 41(2):239−244
Dequeker E, Ligtenberg MJ, Vander Borght S et al (2011) Mutation analysis of KRAS prior to targeted therapy in colorectal cancer: development and evaluation of quality by a European external quality assessment scheme. Virchows Arch 459(2):155–160
Santini D, Loupakis F, Vincenzi B et al (2008) High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist 13(12):1270–1275
Albanese I, Scibetta AG, Migliavacca M et al (2004) Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun 325(3):784–791
Fukunari H, Iwama T, Sugihara K et al (2003) Intratumoral heterogeneity of genetic changes in primary colorectal carcinomas with metastasis. Surg Today 33(6):408–413
Baldus SE, Schaefer KL, Engers R et al (2010) Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res 16(3):790–799
Watanabe T, Kobunai T, Yamamoto Y et al (2011) Heterogeneity of KRAS status may explain the subset of discordant KRAS status between primary and metastatic colorectal cancer. Dis Colon Rectum 54(9):1170–1178
Acknowledgments
We thank Dr. Hitoshi Kanno for kindly providing the data obtained using the KRAS mutation detection kit.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kaneko, Y., Kuramochi, H., Nakajima, G. et al. Degraded DNA may induce discordance of KRAS status between primary colorectal cancer and corresponding liver metastases. Int J Clin Oncol 19, 113–120 (2014). https://doi.org/10.1007/s10147-012-0507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0507-4