Abstract
Purpose
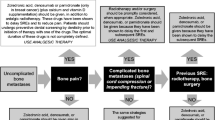
Bone metastases are often asymptomatic and are not diagnosed until after the onset of bone pain. However, bone structural integrity may have diminished considerably before pain onset, resulting in increased risk of skeletal-related events. Therefore, we evaluated whether bisphosphonate therapy was differentially beneficial depending on initiation before or after the onset of bone pain.
Methods
Exploratory analyses were performed in patients with bone metastases from breast cancer or lung cancer/other solid tumors enrolled in two randomized trials comparing monthly zoledronic acid versus pamidronate (breast cancer) or placebo (lung cancer/other solid tumors). Analyses included proportion of patients with one or more skeletal-related events, time to first skeletal-related event, and skeletal morbidity rate in patients with and without baseline pain.
Results
Approximately 80 % of patients reported baseline pain. Similar to overall trial results, zoledronic acid reduced the skeletal morbidity rate in all groups. Although some subsets lacked statistical power, benefits were generally greater in patients without baseline pain. For example, in breast cancer, zoledronic acid increased the 25th quartile of time to first skeletal-related event versus pamidronate by 522 days in patients with no baseline pain (median not reached for either group), but by only 10 days in patients with baseline pain. Similar trends were observed in lung cancer.
Conclusions
Benefits from zoledronic acid appeared to be greater if introduced before bone pain onset. Early diagnosis and treatment of bone metastases may delay onset of skeletal-related events.


Similar content being viewed by others
References
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Coleman RE (2008) Risks and benefits of bisphosphonates. Br J Cancer 98:1736–1740
Lipton A, Theriault RL, Hortobagyi GN et al (2000) Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer (Phila) 88:1082–1090
Saad F, Gleason DM, Murray R et al (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96:879–882
Rosen LS, Gordon D, Tchekmedyian NS et al (2004) Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with non-small cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer (Phila) 100:2613–2621
Saad F, Lipton A, Cook R et al (2007) Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer (Phila) 110:1860–1867
Weinfurt KP, Li Y, Castel LD et al (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16:579–584
Weinfurt KP, Castel LD, Li Y et al (2004) Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care 42:164–175
Sabino MA, Mantyh PW (2005) Pathophysiology of bone cancer pain. J Support Oncol 3:15–24
Heidenreich A, Aus G, Bolla M et al (2008) EAU guidelines on prostate cancer. Eur Urol 53:68–80
Aapro M, Abrahamsson PA, Body JJ et al (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 19:420–432
Van Poznak CH, Temin S, Yee GC et al (2011) American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 29:1221–1227
De Marinis F, Eberhardt W, Harper PG et al (2009) Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J Thorac Oncol 4:1280–1288
Bagi CM (2005) Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev 57:995–1010
Amgen Inc. (2010) Xgeva (denosumab) injection (product insert). http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125320s007lbl.pdf. Accessed Sep 2011
Amgen Inc. (2011) Xgeva 120 mg solution for injection (summary of product characteristics). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf. Accessed Sep 2011
Shore ND, Smith MR, Jievaltas M et al (2011) Effect of denosumab versus zoledronic acid in patients with castrate-resistant prostate cancer and bone metastases: subgroup analyses by prior SRE and baseline pain. J Clin Oncol 29:(abstr 4533)
Rosen LS, Gordon D, Kaminski M et al (2003) Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer (Phila) 98:1735–1744
Body JJ, Diel IJ, Lichinitser MR et al (2003) Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 14:1399–1405
Kretzschmar A, Wiege T, Al-Batran SE et al (2007) Rapid and sustained influence of intravenous zoledronic acid on course of pain and analgesics consumption in patients with cancer with bone metastases: a multicenter open-label study over 1 year. Support Cancer Ther 4:203–210
Gralow J, Tripathy D (2007) Managing metastatic bone pain: the role of bisphosphonates. J Pain Symptom Manag 33:462–472
Kohno N, Aogi K, Minami H et al (2005) Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 23:3314–3321
Vogel CL, Yanagihara RH, Wood AJ et al (2004) Safety and pain palliation of zoledronic acid in patients with breast cancer, prostate cancer, or multiple myeloma who previously received bisphosphonate therapy. Oncologist 9:687–695
Cleeland C, Patrick D, Fallowfield L et al (2010) Effects of denosumab vs. zoledronic acid on pain in patients with advanced cancer and bone metastases: an integrated analysis of 3 pivotal trials. Presented at 35th ESMO Congress, Milan (abstract 1248P)
Saad F, Eastham J (2010) Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 76:1175–1181
Stopeck A, Lipton A, Body JJ et al (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28:5132–5139
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Morgan GJ, Davies FE, Gregory WM et al (2010) First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376:1989–1999
Morgan GJ, Child JA, Gregory WM et al (2011) Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol 12:743–752
Polascik TJ, Mouraviev V (2008) Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag 4:261–268
Estilo CL, Van Poznak CH, Wiliams T et al (2008) Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist 13:911–920
Hoff AO, Toth BB, Altundag K et al (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23:826–836
Lipton A, Siena S, Rader M et al (2010) Comparison of denosumab versus zoledronic acid for treatment of bone metastases in advanced cancer patients: an integrated analysis of 3 pivotal trials. Ann Oncol 21:viii380 (abstr 1249P)
Dimopoulos MA, Kastritis E, Bamia C et al (2009) Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 20:117–120
Ripamonti CI, Maniezzo M, Campa T et al (2009) Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol 20:137–145
Van den Wyngaert T, Claeys T, Huizing MT et al (2009) Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Ann Oncol 20:331–336
Hirsh V, Tchekmedyian NS, Rosen LS et al (2004) Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer 6:170–174
Hatoum HT, Lin SJ, Smith MR et al (2008) Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer (Phila) 113:1438–1445
Tchekmedyian NS, Chen YM, Saad F (2010) Disease progression increases the risk of skeletal-related events in patients with bone metastases from castration-resistant prostate cancer, lung cancer, or other solid tumors. Cancer Invest 28:849–855
Acknowledgments
Funding for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Michael Hobert, PhD, ProEd Communications, Inc.®, for medical editorial assistance with this manuscript.
Conflict of interest
Dr. Costa has received honoraria for lectures at symposia sponsored by Novartis Pharmaceuticals and for participating in advisory board meetings on behalf of Novartis Pharmaceuticals and Amgen. Dr. Lipton has received remuneration for serving on speakers’ bureaus for Novartis Pharmaceuticals and Amgen, has served as a consultant/advisor for Novartis and Amgen, and has received research funding from and provided expert testimony for Novartis. Dr. Hadji has received honoraria, unrestricted educational grants, and research funding from the following companies: Amgen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Novartis, Novo Nordisk, Organon, Pfizer, Procter & Gamble, Roche, Sanofi-Aventis, Solvay, and Wyeth. Dr. Chen is an employee of Novartis Pharmaceuticals and owns stock in the company. Dr. Kosmidis has received honoraria for lectures at symposia sponsored by Novartis Pharmaceuticals and for participating in advisory board meetings on behalf of Novartis Pharmaceuticals. Exploratory analyses were supported by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Costa, L., Lipton, A., Hadji, P. et al. Treatment of bone metastases before the onset of pain. Int J Clin Oncol 18, 531–538 (2013). https://doi.org/10.1007/s10147-012-0414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0414-8