Abstract
Background
We have actively carried out cell-free and concentrated ascites reinfusion therapy (CART) for refractory ascites. However, with conventional CART, the membrane becomes clogged after processing about 2 L of cancerous ascites fluid due to the fact that it is rich in cellular and mucous components; it is therefore difficult to process the entire volume of collected ascites.
Methods
We developed KM-CART which includes a membrane cleaning function, and applied it in 73 cases of cancerous ascites, after its basic functions had been evaluated in 11 cases of refractory cancerous ascites.
Results
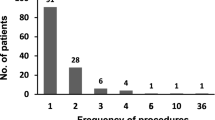
On average, using KM-CART, 6.4 L (range 1.7–14.9 L) of ascites were filtrated and concentrated to 0.8 L (0.2–2.0 L) in 57 min (5–129 min); the membrane was cleaned an average of three times (range 0–10 times) and this enabled the processing of more ascites in a shorter period. In addition, the circuit and the handling were both markedly simple, and fever, which has been the most notable adverse effect with the conventional system, was not an issue.
Conclusion
Since KM-CART was safe and is expected to improve the subjective symptoms and general condition of the patient, it is proposed that this novel system should actively be used not only for palliation but also as supplementary treatment for cancerous peritonitis.



Similar content being viewed by others
References
Britton RC (1961) A new technique for rapid control of cirrhotic ascites. Arch Surg 83:364–369
Inoue N, Yamazaki Z, Sugiyama M et al (1977) Treatment of intractable ascites by continuous reinfusion of the sterilized, cell-free and concentrated ascitic fluid. Trans Am Soc Artif Intern Organs 23:699–702
LeVeen HH, Christoudias G, Moon IP et al (1974) Peritoneo-venous shunting for ascites. Ann Surg 180:580–591
Fukuoka M, Tachibana S, Kimoto T et al (2006) Denver peritoneovenous shunts for palliation of the patient with intractable ascites. J Jpn Surg Assoc 67:575–582
Kao WJ (2000) Evaluation of leukocyte adhesion on polyurethanes: the effects of shear stress and blood proteins. Biomaterials 21:2295–2303
Kitayama J, Ishigami H, Kaisaki S et al (2010) Phase II study of intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 21:67–70
Ishigami H, Kitayama J, Kaisaki S et al (2010) Weekly intravenous and intraperitoneal paclitaxel combined with S-1 for malignant ascites due to advanced gastric cancer. Oncology 78:40–46
Acknowledgments
We thank Wataru Yasui (Department of Molecular Pathology, Hiroshima University Graduate School of Biomedical Sciences) for his valuable advice on the manuscript.
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Japanese CART Study Group., Matsusaki, K., Ohta, K. et al. Novel cell-free and concentrated ascites reinfusion therapy (KM-CART) for refractory ascites associated with cancerous peritonitis: its effect and future perspectives. Int J Clin Oncol 16, 395–400 (2011). https://doi.org/10.1007/s10147-011-0199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0199-1