Abstract
Background
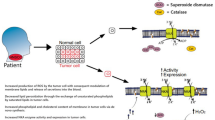
The aim of the study was to investigate the relationships among (a) glutathione peroxidase (GPx) and malondialdehyde (MDA); (b) oncological characteristics (i.e., TNM classification, tumor grade), and; (c) prognosis of head and neck squamous cell carcinoma.
Methods
In a prospective cohort study, we followed 88 patients for 67.4 months (median 40.3) after surgery for head and neck squamous cell carcinoma. Activity of GPx was determined by ELISA and plasma MDA concentration by liquid chromatography.
Results
Lower GPx activity was observed in the T3/4 patients than in the T1/2 group. Tumor grade was significantly correlated with both GPx (P = 0.001) and MDA (P = 0.05, both Spearman). The perioperative level of MDA was higher in patients who later recurred during the follow-up period (n = 15) than in the complete remission group (P = 0.01, Mann–Whitney). Median disease-free interval and overall survival in the group with MDA > median were 29.5 and 32.0 months, respectively, and 38.4 and 40.3 months in the patient group with MDA ≤ median (P = 0.10 and P = 0.08, respectively; Kaplan–Meier). Patients with MDA levels higher than the median had a more than twofold greater risk of recurrence than patients with MDA levels smaller than the median (31.3 vs. 15.2%, P = 0.06, logrank).
Conclusion
Our results suggest that an increased MDA level at the time of initial surgery is found in patients with a high risk of recurrence, which suggests that each patient can be categorized according to risk of recurrence based on their MDA level at the time of initial surgery.




Similar content being viewed by others
Abbreviations
- AOE:
-
Antioxidative enzymes
- CR:
-
Complete remission
- DFI:
-
Disease-free interval
- HNSCC:
-
Head and neck squamous cell carcinoma
- GPx:
-
Glutathione peroxidase
- ery-GPx:
-
Glutathione peroxidase in erythrocytes
- p-GPx:
-
Glutathione peroxidase in plasma
- MDA:
-
Malondialdehyde
- OAS:
-
Overall survival
- ROS:
-
Reactive oxygen species
References
Arikan S, Akcay T, Konukoglu D et al (2005) The relationship between antioxidant enzymes and bladder cancer. Neoplasma 52(4):314–317
Jaloszynski P, Jaruga O, Olinski R et al (2003) Oxidative DNA base modifications and polycyclic aromatic hydrocarbon DNA adducts in squamous cell carcinoma of larynx. Free Radic Res 37(3):231–240
Curtin JF, Donovan M, Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 265:49–72
Subapriya R, Kumaraguruparan R, Nagini S et al (2003) Oxidant–antioxidant status in oral precancer and oral cancer patients. Toxicol Mech Methods 13:77–81
Sabitha KE, Shyamaladevi CS (1999) Oxidant and antioxidant activity changes in patients with oral cancer and treated with radiotherapy. Oral Oncol 35:273–277
Rush JW, Sandiford S (2003) Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem 36:345–351
Ratnasinghe D, Tangrea JA, Andersen MR (2000) Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res 60:6381–6383
Hu YJ, Diamond AM (2003) Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res 63(12):3347–3351
Moscow JA, Morrow CS, He R, Mullenbach GT et al (1992) Structure and function of the 59-flanking sequence of the human cytosolic selenium-dependent glutathione peroxidase gene. J Biol Chem 267:5949–5958
Forsberg L, de Faire U, Morgenstern R (2001) Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys 389(1):84–93
Bohn SK, Smeland S, Sakhi AK (2001) Post-radiotherapy plasma total glutathione is associated to outcome in patients with head and neck squamous cell carcinoma. Cancer Lett 18(2):240–247
Smilek P, Kostřica R, Dušek M et al (2006) Predictive parameters of the head and neck carcinomas. Otorinolaryngologie a Foniatrie 55(1):228–229
Khoschsorur GA, Winklhofer Roob BM, Hl Rabl et al (2000) Evaluation of a sensitive HPLC method for determination of malondialdehyde, and application of the method to different biological materials. Chromatographia 52:181–184
Cox DR (1972) Regression models and life tables. J R Stat Soc Ser B34(2):187–220
Grambsch P, Therneau T (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526
Salzman R, Kaňková K, Pácal L et al (2007) Increased activity of superoxide dismutase in advanced stages of head and neck squamous cell carcinoma with locoregional metastases. Neoplasma 54:321–325
Szuster-Ciesielska A, Hryciuk-Umer E, Stepulak A et al (2004) Reactive oxygen species production by blood neutrophils of patients with laryngeal carcinoma and antioxidative enzyme activity in their blood. Acta Oncol 43(3):252–258
Yigitbasi OG, Guney E, Haghighi N et al (2000) Oxidant and antioxidant status in larynx squamous cell carcinomas. J Exp Clin Cancer Res 19(4):447–451
Bhuvarahamurthy V, Balasubramanian N, Govindasamy S (1996) Effect of radiotherapy and chemoradiotherapy on circulating antioxidant system of human uterine cervical carcinoma. Mol Cell Biochem 158(1):17–23
Korotkina RN, Matskevitch GN, Devlikano AS et al (2002) Activity of glutathione metabolizing and antioxidant enzymes in malignant and benign tumors of human lungs. Bull Exp Biol Med 133(6):606–608
Kaynar H, Meral M, Turhan H et al (2005) Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett 227(2):133–139
Taysi S, Uslu C, Akcay F et al (2003) Malondiadehyde and nitric oxide levels in the plasma of patients with advanced laryngeal cancer. Surg Today 33(9):651–654
Karu I, Taal G, Zilmer K et al (2010) Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand Cardiovasc J 44(2):119–124
Acknowledgments
This project is supported by grant no. 10/II NR/9200-3 from The Internal Grant Agency of The Ministry of Health of the Czech Republic, Prague. The study was conducted in accord with the Helsinki Declaration of 1964 and all subsequent revisions of it, and was approved by the ethical committee of St. Anne’s Faculty Hospital, Brno, Czech Republic.
Conflict of interest statement
None to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Salzman, R., Pácal, L., Kaňková, K. et al. High perioperative level of oxidative stress as a prognostic tool for identifying patients with a high risk of recurrence of head and neck squamous cell carcinoma. Int J Clin Oncol 15, 565–570 (2010). https://doi.org/10.1007/s10147-010-0108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0108-z