Abstract
Background
This study aimed to evaluate the tolerability and pharmacokinetics of capecitabine, given twice daily for 6 weeks without interruption, and to identify the maximum tolerated dose (MTD) and the suggested phase II schedule.
Methods
The initial dose of capecitabine was 251 mg/m2 twice daily, without interruption, and a dose escalation schedule was planned according to a modified Fibonacci scheme. Patients received oral capecitabine twice daily for at least 6 weeks unless grade 3 or 4 toxicity was observed. Blood and urine samples were collected for pharmocokinetic analysis on days 1 and 15.
Results
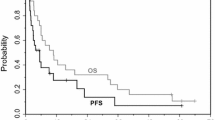
Sixteen patients with malignant solid tumors (seven breast, seven colorectal, and two gastric) were enrolled, all of whom were evaluable. Among 4 patients treated with capecitabine 1255 mg/m2 twice daily, one experienced grade 4 hemorrhagic gastric ulcer and one experienced grade 3 skin toxicity. Consequently, this dose was defined as the MTD, and gastrointestinal and cutaneous effects were identified as dose-limiting toxicities. There was no grade 3/4 diarrhea at any dose level. There was also no grade 4 hematologic toxicity at doses below the MTD, and there were no treatment-related deaths. Two patients with breast cancer had partial responses, at capecitabine doses of 502 mg/m2 and 1255 mg/m2, twice daily. Pharmacokinetic data show that high concentrations of doxifluridine (5′-DFUR) occur in tumor cells within 2 h after administration of capecitabine.
Conclusion
The MTD of continuous oral capecitabine over 6 weeks was 1255 mg/m2 twice daily. Because of the higher occurrence of skin disorders reported in this study with a continuous treatment regimen, an intermittent treatment regimen of 828 mg/m2, administered twice daily for 3 weeks, followed by a 1-week rest period, was recommended for phase II studies. This regimen is being evaluated in phase II studies in Japanese patients with gastric, colorectal, and breast cancers.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Saeki, T., Takashima, S., Terashima, M. et al. A Japanese phase I study of continuous oral capecitabine in patients with malignant solid tumors. Int J Clin Oncol 10, 51–57 (2005). https://doi.org/10.1007/s10147-004-0460-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10147-004-0460-y