Abstract
Cervical spondylotic myelopathy (CSM) is a degenerative disease representing the most common spinal cord disorder in the adult population. It is characterized by chronic compression leading to neurological dysfunction due to static and dynamic injury of the spinal cord in cervical spine. These insidious damage mechanisms can result in the reorganization of cortical and subcortical areas. The cerebral cortex can reorganize due to spinal cord injury and may play a role in preserving neurological function. To date, the gold standard treatment of cervical myelopathy is surgery, comprising anterior, posterior, and combined approaches. However, the complex physiologic recovery processes involving cortical and subcortical neural reorganization following surgery are still inadequately understood. It has been demonstrated that diffusion MRI and functional imaging and techniques, such as transcranial magnetic stimulation (TMS) or functional magnetic resonance imaging (fMRI), can provide new insights into the diagnosis and prognosis of CSM. This review aims to shed light on the state-of-the-art regarding the pattern of cortical and subcortical areas reorganization and recovery before and after surgery in CSM patients, underlighting the critical role of neuroplasticity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical spondylotic myelopathy (CSM) is a comprehensive definition used to describe a complex of age-related and progressive conditions affecting the cervical spine structures, ultimately leading to chronic compression of the cervical spinal cord [1]. It is adults’ leading cause of spinal cord injury [2]. Based on data for the general population, CSM affects approximately 10% of patients over 50 years old. Notably, even though more than 50% of individuals show radiologic evidence of spondylosis of the cervical spine tract, only a fraction of the patients tends to develop clinical signs [3]. Degeneration involves the intervertebral disc and the capsule-ligamentous and bony compartments of the cervical spine. These pathological modifications lead to the spinal canal and foramen’s narrowing, eventually resulting in neural structure compression [2, 4, 5]. Long-term compression of the cervical spinal cord can cause the degeneration of the anterior horn and motor neurons, even the lateral and posterior funiculus axons demyelination.
Typical findings include limb numbness and bilateral fine motor deficits with loss of dexterity, hemi- or quadriparesis, ataxic gait, sphincter disturbances, hyperreflexia, and clonus [6]. Treatments include conservative management, such as neck immobilization, pharmacologic therapies, lifestyle modifications, physical modalities, and surgical options [7].
The goal of surgery is the expansion of the spinal canal to provide a decompression, improve spinal cord morphology, and achieve a successful fusion, thus preventing the development of late deformity.
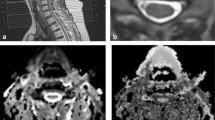
There are many options in the operating room, including anterior cervical discectomy and fusion (ACDF), which represents one of the most commonly performed surgical procedure in CSM, firstly described by Robinson and Smith in 1955 and by Cloward in 1958 [8, 9] (Fig. 1A); corpectomy [10, 11]; laminoplasty that allows for avoiding the implant of vertebral screws [12]; and laminectomy (with or without fusion), the procedure of choice in multiple-level compression in patients with preserved cervical lordosis. The latter especially indicated in elderly patients, where comorbidities increase the operative risk [13,14,15] (Fig. 1B).
Finally, it is possible to perform combined approach, particularly indicated for patients with severe kyphotic angulation necessitating decompression of 3 or more levels [16].
To date, decompressive surgery remains the most effective long-term treatment for this pathology, although the decision of which type of approach use and when to perform such a procedure remains challenging [17].
Several prognostic factors could affect the outcome after surgical management [18]. The most important prognostic factors are duration of symptoms, preoperative neurological status, the effective diameter of the canal, number of compression levels, intrinsic cord alterations (assessed from pre-operative MRI study), and other clinic-radiological features [3, 19,20,21,22]. In recent years, novel techniques have been applied to investigate central nervous system (CNS) pathology [23, 24].
For instance, it has been shown that diffusion weighted imaging (DWI), tractography, fMRI, and other functional techniques are more valuable than routine MRI scans for diagnosis and predicting outcomes in CSM patients [25, 26]. DTI-tractography and diffusion MRI (dMRI) techniques in general allow the mapping of neural connections by tracking water movement across axons while simultaneously measuring important metrics like fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusion (RD) [27, 28]. FA quantifies how much directional coherence exists between tissues — giving an insight into nerve tract connectivity quality and correlating with tissue integrity [29]. Instead, MD values in brain regions indicate the water diffusion degree, and higher values are often associated to brain disease. Conversely, AD and RD reflects other features related to axonal structure and integrity [30, 31]. On the other hand, functional MRI-based imaging technique is based on the analysis of the BOLD (blood oxygen level-dependent) signal, which in turn estimates changes in blood oxygenation in response to neural activity or at rest (the so-called resting state fMRI or rsfMRI) [32]. Particularly important are the spatial and temporal resolution of the signal and the VOA, which need to be carefully known and interpreted [33,34,35]. Finally, TMS represents a noninvasive brain stimulation technique used to induce excitability/inhibition changes in the cerebral cortex through a coil that generates a magnetic field. It has become a fundamental tool for diagnostic purposes to mapping brain functioning in humans (the so called navigated TMS, or nTMS) and for therapeutic ones to induce neuroplasticity phenomena (repetitive TMS, or rTMS) [36]. Several TMS stimulation paradigms exist, such as short interval cortical inhibition (SICI), intracortical facilitation (ICF), long interval cortical inhibition (LICI), and paired associative stimulation (PAS), each with its own peculiarities and indications [37,38,39].
Despite these advanced tools, only a few determinants of functional recovery have been defined [40, 41]. The complex physiologic recovery processes involving cortical and subcortical neural reorganization following surgery are still inadequately understood. To assess the functional recovery of the central nervous system (CNS) following CSM surgery, many diagnostic imaging techniques, including fMRI, PET, TMS, diffusion tensor imaging tractography, and other functional studies, have been proposed [40,41,42]. Neuronal plasticity affects structural and functional reorganization of either brain or spinal cord fiber tracts, allowing, over time, for compensation of the previously established neurological deficits [43, 44]. To date, there are still no validated scores analyzing pre- and postoperative supraspinal functional reorganization, with the goal of stratifying patients prognostically, trying to understand which variables and factors are involved in neuroplasticity and how they influence the recovery process.
This review aims to evaluate the state-of-the-art about pattern of cortical reorganization and recovery of the CNS after surgery in CSM patients and to find possible prognostic factors associated with the types of cortical reorganization.
Materials and methods
Search of the literature
Preferred reporting items for systematic reviews and meta-analyses guidelines (PRISMA) were followed to conduct and report this systematic literature review (Fig. 2) [45]. We performed a broad systematic literature search in Pubmed for all studies investigating neuroplasticity associated to cortical reorganization in patients with cervical myelopathy undergoing surgery. We searched for studies published up to the 17th of May 2022 without backward limits, using the following MeSH and free text terms “cervical myelopathy”, “functional reorganization”, “white matter tracts”, “FMRI”, “tractography”, “cortical reorganization”, and “surgery”, combined using Boolean operator “AND”. To avoid the potential omission of relevant studies, we also manually screened reference lists of articles included and previous systematic reviews and meta-analyses regarding cervical myelopathy, surgery, and cortical functional reorganization and neuroplasticity. Duplicate articles were eliminated using Microsoft Excel 16.37.
Study selection
The research strategy initially relied on title and abstract analysis. The article’s full text was retrieved for further investigation if the title and abstract met the inclusion criteria. The data collection process was conducted without using any automated tools. No ethical approval was required for this study.
Eligibility criteria
The articles were selected according to the following inclusion criteria:
-
Full article in English
-
Case report, case series, retrospective study, and prospective study
-
Patients age ≥ 18
-
Patients affected by cervical myelopathy (defined clinically by standardized scales such as the mJOA or radiologically, as an area of hyperintensity at the level of the medullary cord in T2-weighted MRI sequences).
-
Patients treated by surgery with subsequent investigation of cortical functional reorganization and neuronal plasticity.
Exclusion criteria:
-
Articles not in English
-
Editorials, books, systematic reviews, and meta-analysis
-
Patients age < 18
-
Patients treated without surgery.
-
Studies not evaluating cortical neuronal plasticity in the pre- and post-operative period.
-
Studies in a preclinical phase (animal study or in vitro study)
Data extraction
According to the criteria above, all articles were identified by two reviewers (L.C and M.P). In case of a discrepancy, a third author (L.B) arbitrated until a consensus among the authors was reached.
The extracted data included the following: publication’s year, author, patients’ number, country, study design, postoperative clinical improvement (assessing through post-op mJOA score), methodic, area of interest, postoperative vs. preoperative comparison, post-operative vs. control comparison, others, and analyzed value.
Statistical analysis
Microsoft Excel – 2022 and Prism – GraphPad 2022 have been used for statistical analyses. Means and SDs or medians and ranges were calculated for continuous variables depending on the distribution of the variable. Frequencies and percentages were calculated for nominal variables. A 2-sided χ2 test or an independent 2-tailed and unpaired t test was performed to test differences between patients and control group or preop mJOA and postop mJOA. Only results with a p value < 0.05 were considered statistically significant.
Results
Data selection
Our initial research through Pubmed identified a total of 468 articles. We excluded 35 duplicated articles, then performed further screening based on title and abstract reading, eliminating 396 articles.
Finally, after a full-text reading and a detailed examination, we included 13 studies in our systematic review, according to PRISMA flow diagram inclusion criteria.
The characteristics of included articles are shown in Table 1. Data extracted were the number of patients, age, gender, the control group characteristics, pre-, and postoperative mJOA scores, methods used, investigated area, differences between control group, pre-, and postoperative patients.
Patient’s demographic data and study characteristics
We have analyzed a total number of 240 patients, which mean age was 51.97 ± 6.84 years. This cohort has a male prevalence compared to female (74.16% vs. 25.83%).
As shown in Table 1, among our selected studies, three papers analyzed the role of DTI in cervical myelopathy, eight papers fMRI, one article on other tools associated with MRI, and one paper on transcranial magnetic stimulation to evaluate MEP. Eleven of all the works included in our review had a study design including a control group cohort.
Clinical and demographic data of our selected cohort are also reported in Table 1.
Data from patients who undergone to fMRI had shown that, when motor area was chosen as ROI, there was a reduction of postoperative FC of approximately the 87.5% compared to preoperative; in all cases (100%), it has been shown a higher value for preoperative FC in comparison to control group. When ROI was put on the sensory area, it was enlightened that postoperative VOA had a lower value than preoperative VOA in the 50% of cases and preoperative VOA had a lower value than controls in the 50% cases.
In patients who have undergone DTI-tractography, it has been shown in one study that postoperative neurological improvement was more common in patients with intact fibers, in another study that postoperative mJOA tends to a positive correlation to postoperative FT and in the third study that preoperative FA and ADC values have a statistically significant correlation to postoperative mJOA.
In all studies evaluating the neurological status by mJOA score, a statistically significant difference has been shown between preoperative mJOA and post-operative mJOA, underling a beneficial effect of decompressive surgery (p < 0.001).
Discussion
The concept of neuroplasticity
CSM is a debilitating condition characterized by neurological impairment due to static and dynamic injury of the spinal cord in the cervical spine [55]. Chronic spinal cord compression produces necrosis and demyelination of gray and white matter, resulting in progressive neurological impairment associated with motor, sensory, and autonomic disability [1].
In this context, it has been demonstrated that neural plasticity of the cortical network allows for the minimization of functional impairment. It can occur via synaptic modification of preexisting connections or by developing new circuitry [56,57,58]. Neuronal plasticity allows neurons in the brain and spinal cord to compensate for injury by adjusting their activities or structures in response to new situations [59]. The cellular correlates underlying neuronal plasticity involve morphological and functional adaptations in synapses, dendrites, and axons. These mechanisms result in a change in the input–output function of the neural network and, therefore, in a modification of information processing [60, 61] (Fig. 3).
Schematic representation of the neuronal plasticity and cellular processes involved. Schematic representation of the neuronal plasticity mechanisms: enhanced synaptic strength; increased dendritic excitability facilitates synaptic integration and neuronal firing; enhanced cellular excitability results in a lower threshold for potential action generation; altered connectivity pattern results in neuronal network modulation and increased neuronal activity
Contrary to what is postulated by the theory of localizationism, whereby each part of the brain is dedicated to a specific function and operates independently of the other portions, the concept of neuronal plasticity is based on the idea that the brain and spinal cord, when faced with events disrupting their normal physiology, can build up or adapt themselves to variable or persistent demands to limit the damage and safeguard the injured function [62, 63]. Cornerstones of neuroplasticity are modularity, redundancy, and distributed processing [64]. Modularity is the organization of a system into modules. Each module performs a discrete function, and if one module is eliminated selectively, the remaining modules can sustain the global role of the system. Otherwise, redundancy ensures the persistence of a function even after the brain’s partial destruction, thus indicating that distinctly different elements may sustain the same function [65, 66]. From an anatomophysiological point of view, processes involving neural plasticity include neurogenesis, cell migration, changes in neuronal excitability and neurotransmission, the generation of new connections, and modification of existing ones [67, 68].
Tools for evaluating cortical functional reorganization
The dorsal column of the spinal cord is composed of sensory afferent fibers. Due to an injury, the section of this pathway in the cervical spinal cord can deactivate much of the primary somatosensory cortex and other areas. Following this injury, these affected areas can go through a reorganization to ensure a functional recovery by creating new neuronal circuitry [69]. Over the years, several studies focused on the concept of neural plasticity following cervical injury, the imaging techniques to evaluate it, and the relation between preoperative and postoperative imaging findings and the functional recovery of patients after surgery.
For instance, it is possible to employ functional MRI (fMRI) to evaluate neural plasticity. In particular, the differentiation due to acute spinal cord injury causes an immediate change of wide cortical networks that can be noticed on fMRI, which shows the expansion of M1 representation and the increased activation of supplementary motor areas as the result of the cerebral reorganization [40, 70].
Duggal et al. [47] compared preoperative and postoperative brain fMRI to characterize the changes occurring in cortical activation after surgical decompression: in preoperative patients, the volume of activation (VOA) was greatest compared to controls within the precentral gyrus, while VOA was greater after surgical decompression, a characteristic which may represent increased recruitment within the primary motor cortex in patients with cervical myelopathy; instead, within the postcentral gyrus, VOA was greater in controls, which may reflect a degree of local cortical atrophy. The degree of VOA at fMRI is still a matter of debate, since it plays a divisive role, marking a difference between various included studies’ results; it seems clear that there are studies in which VOA is increased, between preoperative and postoperative evaluation, and others where is decrease. Sawada et al. [48] have tried to explain this contradiction referring to the absence of an in-depth study of the relationship between task difficulty and recruited brain region, used in the assessment of VOA. Bhagavatula et al. [43] switch the focus on the heterogeneity of patients with CSM which present with different degrees of spinal cord impairment and they reflected on how the extent of synaptic transmission and reorganization depend on the time elapsed since the initial insult and the heterogeneity of residual spinal cord atrophy. All these topics suggest that there is still no simple and unambiguous way to interpret these data and further studies on this topic are needed.
From a structural connectivity point of view, DTI-tractography is an MRI technique that allows the noninvasive measurement of the translational motion of water, providing information such as the FA in different tissues and measuring their integrity. Several studies reported changes in parameters in patients affected by cervical myelopathy compared to healthy controls, especially in the FA values, which seem to be strictly related to recovery rates in patients affected by cervical myelopathy, while the high signal intensity of the spinal cord on T2WI does not. Thus, FA, and DTI-tractography, can be used as objective prognostic factors to predict the patient clinical outcome and the rate of functional recovery after decompression surgery [42, 54, 71]. However, DTI-tractography has some intrinsic limitations. First, it is not sensitive to the complex architecture of cortical white matter, particularly it cannot directly image multiple fiber orientations within a single voxel; the reason for this is that the tensor model approaches fiber orientation with an ellipsoid shape. In a region where fibers cross, the orientation estimation of the tensor model will approach a sphere and thus cannot capture the orientation of two separate fibers [72]. To overcome this drawback various algorithms have been proposed, including constrained spherical deconvolution (CSD) [73], spherical-deconvolution informed filtering of tractograms (SIFT) [74], or multi-shell multi-tissue CSD (MSMT) [75]
Another possible technique is magnetic resonance spectroscopy (MRS), which can detect metabolites and study tissue biochemistry in vivo. In CSM patients, MRS can identify a decreasing inN-acetylaspartate (NAA) to creatine (Cr) ratio in the primary motor cortex after injury. These alterations suggest neuronal impairment or altered energy metabolism and have proven to be strictly related to changes in mJOA scores after surgery [76].
DTI and MRS can be used in combination to provide a stronger correlation between the patients’ symptoms, assessed through the mJOA score, and the degree of neurological impairment. Thanks to the evaluation of DTI parameters (e.g., FA) and MRS biomarkers (e.g., Cho/NAA ratio), it is possible to assess the microstructural and metabolic patterns following the cervical injury and better define the post-surgical functional status [77].
Recently, navigated transcranial magnetic stimulation (nTMS) has proven to be a valuable tool in characterizing functional impairment in patients affected by cervical myelopathy. Indeed, some neurophysiological measurements, such as the corticospinal excitability, rate of inhibition, and motor area behavior, are related to the symptomatology and the clinical course. The detection of the degree of compensatory reorganization given by these parameters can be therefore used to stratify patients in terms of risk for further neurological deterioration [70]. nTMS can also be used after decompression to demonstrate the compensatory expansion of motor cortex, to identify patient potentially recovery and the correct rehabilitative program after surgery [51].
The articles included in our review point out the remarkable usefulness of such techniques for diagnostic and, mostly, prognostic purposes due the opportunity to obtain information about the functional aspect of the injured cervical spinal cord, and the cortico-subcortical reorganization of particular brain areas. However, even though they are currently employed in the clinical practice, they do not belong to standardized diagnostic algorithms and are a prerogative of few specialized units.
The effect of surgery in cortical areas reorganization in cervical myelopathy
It has been observed that neck disability and symptom severity in cervical myelopathy can be related to brain alterations in both cortical microstructure and functional connectivity [78], such as the decrease in cortical thickness and the increase in functional connectivity between primary sensorimotor and supplemental motor or sensory regions. Consistent with these observations, Wang et al. [57] observed significant alterations in white matter tracts connecting primary motor and sensory cortices using diffusion spectrum imaging (DSI). This method, unlike classical DTI-tractography, is able, through special acquisition protocols and post-processing software, to detect the orientation of multiple fibers within a voxel, allowing the delineation of fibers that cross and touch in the brain. This technique is based on the study of the probability density function (PDF) that for each voxel specifies the 3D distribution of microscopic spin displacements visible in MR that it contains [79]. Given the ability to accurately display the structural changes of fiber tracts, DTI based on DSI has already been used in some neurodegenerative and mental disorders, with promising results [80, 81].
Aleksanderek and colleagues [49] compared the functional reorganization in the primary motor cortex in patients affected by mild and moderate CSM by using the MR spectroscopy. They found that, before surgery, the NAA/Cr ratio was lower in patients with mild CSM, compared to healthy control patients and subjects with moderate CSM. Following surgery, both the groups demonstrated a functional improvement, and six months after surgery, the NAA/Cr ratio decreased significantly in patients affected by moderate CSM. The neurological recovery observed can thus be explained by the mitochondrial and synaptic dysfunction, evidenced by low levels of NAA/Cr, which represent the primary trig for cortical reorganization.
Cortical changes occurring after surgery were also evaluated by Bhagavatula et al. [43] by using the blood oxygen level-dependent (BOLD) functional MRI (fMRI): after CSM, to compensate for motor weakness and loss of dexterity, there is over-recruitment of sensorimotor cortices (left precentral gyrus) documented by an VOA, compared to the control group. Postoperatively, in response to surgery, the cortical reorganization is demonstrated by activating and recruiting other areas, such as the premotor and supplementary motor areas. This recruitment can be already observed six months after surgery and can be relevant for the maintenance of function after injury and in the recovery process itself [50].
Functional reorganization is typical in CSM patients localized to sensorimotor, regulatory, and visual processing regions [82]. Indeed, after CSM the sensory components transmitted to the thalamus can decrease, thus leading to alterations in thalamus-cortex circuits. Through fMRI, it is possible to study the alterations in functional connectivity values between the thalamus and the cortex. The abnormal values observed after the cervical injury (increased compared to healthy controls) demonstrate that spinal damage has an impact on brain activity and can be used as biomarkers to assess neuronal damage and predict patient outcomes; moreover, the altered functional connectivity observed in post-surgery patients (which is decreased compared to healthy controls) is suggestive for the adaptive changes occurring after decompression [46]. Also, the brainstem and the cerebellum lobes, evaluated through fMRI, showed to play a relevant role in the pathway of functional brain reorganization in patients affected by cervical injury [83].
Prognostic factors related to neural plasticity in CSM
Strategies to predict neurological recovery and clinical outcomes following decompression surgery in patients affected by CSM are needed. The current prediction is based on multiple factors: age, duration of symptoms, preoperative neurological status, and radiological findings, such as signs of instability or T2 high-intensity signals on MRI [84, 85]; on the contrary, the type of approach chosen by the surgeon, both ventral or dorsal, does not seem to affect the prognosis [86]. However, a prediction based on these factors remains subjective and not quantitative [87].
In patients affected by CSM, functional alterations can be observed in the visual cortices through fMRI and these alterations are strictly related to visual acuity. Zhou et al. [88] observed that patients show higher bidirectional effective connectivity between the secondary visual cortex and the cerebellum in CSM patients, and this increase seems to be related to the prognosis. Consequently, structural alterations and adaptive changes in patients suffering from myelopathy play a crucial role in neuropathology.
To define the relation between functional reorganization and clinical outcome, thus identifying prognostic markers, the functional connectivity (FC), a technique used to identify spatial patterns of coherent BOLD activity, can be used. According to literature, an increased FC between visual associated brain regions and the cerebellum is related to altered visual function and impaired motor function, therefore negatively related to JOA scores in patients affected by CSM [89]; accordingly, FC may act as a potential biomarker for postoperative gain and potential recovery [90]. The association between FC and prognosis could also be explored via support vector regression (SVR), using preoperative FC as features and JOA recovery rate as labels. Predicting CSM-related outcomes through machine learning techniques is not yet robust enough to be used in clinical practice, and so further studies are needed [91].
Another fMRI technique used to predict the prognosis of patients is the multivariate pattern analysis, which evaluates the static amplitude of low-frequency fluctuation (sALFF) and the dynamic amplitude of low-frequency fluctuation (dALEF). Brain regions that successfully predicted the clinical outcome were mainly located at the frontal cortices for sALFF and frontal cortices, left insular, and posterior lobe of the cerebellum for dALFF. Therefore, the functional alterations documented by the static and dynamic ALFF are related to patient’s clinical outcome and can be employed to determine prognostic biomarkers for cervical myelopathy [92].
Moreover, CSM patients exhibit a higher ALFF within left motor cortex and bilateral superior frontal gyrus and lower zALFF within right precuneus and calcarine; the functional status of M1 contributes to the severity of CSM, and thus the M1 zALFF can be considered a valuable predictor of the prognosis in CSM patients after decompression surgery, given that patients with more severe symptoms show this pattern on fMRI [93]. Moreover, preoperatively increased ALFF decreases after surgery in the primary sensorimotor cortex and visual cortex [87].
Craciunas et al. [94] found that the postoperative clinical status is strictly associated with preoperative levels of specific metabolites across M1 and the cerebellum. Specifically, preoperative levels of myo-inositol and glutamate–glutamine in the cerebellum were associated with the extent of lower extremity disability. In contrast, higher levels of the upper-extremity sensorimotor function were related to higher levels of NAA and glutamate–glutamine in the left M1. These findings suggest that patients with less neuroinflammation and neuronal metabolic depression have a higher potential for functional reorganization.
Limitations
Given the studies heterogeneity and the small number of samples examined, the picture rendered to the reader appears to be a general overview of the functional and structural connectivity imaging and stimulation techniques that are increasingly being employed in this setting and their potential. In this context, our study presents some limitations. First, the paucity of studies in this regard and the small sample size examined do not allow generalization of the results, further leading to the difficulty in translating the results obtained into daily clinical practice. Secondly, the studies included in this review consider different methods in assessing the functional reorganization of cortical networks, making interpreting results and their applicability for prognostic purposes difficult. Third, another limitation is the nonunique definition of cervical myelopathy. In fact, the diagnosis of CSM is made using clinical criteria, radiological criteria, or both. To overcome this obstacle, the use of standardized clinico-radiological scores would certainly help to quantitatively implement the inherently qualitative definition of cervical myelopathy. Finally, further longitudinal studies appear to be necessary to evaluate the effect of decompression surgery on alterations of dynamic connectomics of brain networks. Moreover, the lack of standardized acquisition protocols results in a potential risk of bias in the analysis of results obtained even with identical tools. This last point can only be overcome by introducing these techniques into clinical practice and routine evaluation of the patient with cervical myelopathy, adopting standardized algorithms to improve the collection and analysis of the data obtained also from different centers.
Conclusions
CSM is a subtle and debilitating condition resulting in various neurological deficits. Neural plasticity of the cortical network allows functional impairment to be minimized. Several imaging modalities exist to assess the functional status and postoperative reorganization of the neural network in patients affected by CSM. These techniques could be helpful in more accurately predicting the outcome of patients undergoing cervical surgery. Our review represents a first effort to unify the results obtained so far in this new and still under-explored field. Much ground remains to be covered in understanding the precise role of each of these tools, the data they provide, and their usefulness in clinical practice.
Nonetheless, surveying the relationship between CSM and the plasticity of cortical areas and white matter fibers after surgery appear worthy of investigation, so further studies could be needed to explore this topic in-depth and unify the findings obtained. Future directions should first evaluate the clinical applicability of the results obtained to stratify patients from a prognostic point of view. In addition, researchers should analyze possible differences in the reorganization of cortical areas according to the surgical approach used, carrying out the comparison of CSM patients before and after surgery and long-term follow-up after surgery for a better comprehension of functional reorganization patterns.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Tracy JA, Bartleson BJ (2010) Cervical spondylotic myelopathy. Neurologist 16:176–187. https://doi.org/10.1097/NRL.0b013e3181da3a29
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 40:E675–E693. https://doi.org/10.1097/BRS.0000000000000913
Iyer A, Azad TD, Tharin S (2016) Cervical spondylotic myelopathy. Clin Spine Surg 29:408–414. https://doi.org/10.1097/BSD.0000000000000397
Shedid D, Benzel EC (2007) Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 60:S1
Baron EM, Young WF (2007) Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 60. https://doi.org/10.1227/01.NEU.0000215383.64386.82
Harrop JS, Hanna A, Silva MT, Sharan A (2007) Neurological manifestations of cervical spondylosis: an overview of signs, symptoms, and pathophysiology. Neurosurgery 60. https://doi.org/10.1227/01.NEU.0000215380.71097.EC
Bakhsheshian J, Mehta VA, Liu JC (2017) Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J 7:572–586
Cloward RB, Honolulu Md (1958) The anterior approach for removal of ruptured cervical disks. J Neurosurg. https://doi.org/10.3171/jns.1958.15.6.0602
Burkhardt BW, Brielmaier M, Schwerdtfeger K, Sharif S, Oertel JM (2017) Smith-Robinson Procedure with and without caspar plating as a treatment for cervical spondylotic myelopathy: a 26-year follow-up of 23 patients. Eur Spine J 26:1246–1253. https://doi.org/10.1007/s00586-017-4988-8
Perez-Cruet MJ, Samartzis D, Fessler RG (2006) Anterior cervical discectomy and corpectomy. Neurosurgery 58(4 Suppl 2):ONS-355-9. https://doi.org/10.1227/01.neu.0000205285.20336.c2
Jiang SD, Jiang LS, Dai LY (2012) Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion for multilevel cervical spondylosis: a systematic review. Arch Orthop Trauma Surg 132:155–161
Cho SK, Kim JS, Overley SC, Merrill RK (2018) Cervical laminoplasty: indications, surgical considerations, and clinical outcomes. J Am Acad Orthop Surg 26:e142–e142. https://doi.org/10.5435/JAAOS-D-16-00242
Cole T, Veeravagu A, Zhang M, Azad TD, Desai A, Ratliff JK (2015) Anterior versus posterior approach for multilevel degenerative cervical disease: a retrospective propensity score-matched study of the MarketScan Database. Spine (Phila Pa 1976) 40:1033–1038. https://doi.org/10.1097/BRS.0000000000000872
Lawrence BD, Jacobs WB, Norvell DC, Hermsmeyer JT, Chapman JR, Brodke DS (n.d.) Anterior versus posterior approach for treatment of cervical spondylotic myelopathy A systematic review. Number 22S(38):173–182. https://doi.org/10.1097/BRS.ObOI
Youssef JA, Heiner AD, Montgomery JR, Tender GC, Lorio MP, Morreale JM, Phillips FM (2019) Outcomes of posterior cervical fusion and decompression: a systematic review and meta-analysis. Spine J 19:1714–1729. https://doi.org/10.1016/j.spinee.2019.04.019
Kim PK, Alexander JT (2006) Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J 6. https://doi.org/10.1016/j.spinee.2006.04.025
Gerardi RM, Giammalva GR, Basile L, Gulì C, Pino MA, Messina D, Umana GE, Graziano F, di Bonaventura R, Sturiale CL et al (2022) White cord syndrome after cervical or thoracic spinal cord decompression. hemodynamic complication or mechanical damage? An Underestimated Nosographic Entity. World Neurosurg 164:243–250. https://doi.org/10.1016/j.wneu.2022.05.012
Giammalva GR, Maugeri R, Graziano F, Gulì C, Giugno A, Basile L, Iacopino DG (2017) White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF): a “No Reflow Phenomenon”? Interdisc Neurosurg 7:47–49. https://doi.org/10.1016/j.inat.2016.12.001
Ramesh V, Kannan MV, Sriram K, Balasubramanian C (2017) Prognostication in cervical spondylotic myelopathy: proposal for a new simple practical scoring system. Asian J Neurosurg 12:525. https://doi.org/10.4103/1793-5482.146391
Park JH, Kim JH, Roh SW, Rhim SC, Jeon SR (2017) Prognostic factor analysis after surgical decompression and stabilization for cervical spinal-cord injury. Br J Neurosurg 31:194–198. https://doi.org/10.1080/02688697.2016.1247781
McCormick JR, Sama AJ, Schiller NC, Butler AJ, Donnally CJ (2020) Cervical spondylotic myelopathy: a guide to diagnosis and management. J Am Board Fam Med 33:303–313. https://doi.org/10.3122/jabfm.2020.02.190195
Aggarwal R, Srivastava S, Bhosale S, Nemade P (2016) Prediction of surgical outcome in compressive cervical myelopathy: a novel clinicoradiological prognostic score. J Craniovertebr Junction Spine 7:82. https://doi.org/10.4103/0974-8237.181828
Tu J, Castillo JV, Das A, Diwan AD (2021) Degenerative cervical myelopathy: insights into its pathobiology and molecular mechanisms. J Clin Med 10:1–28. https://doi.org/10.3390/jcm10061214
Wang K, Zhu S, Mueller BA, Lim KO, Liu Z, He B (2008) A new method to derive white matter conductivity from diffusion tensor MRI. IEEE Trans Biomed Eng 55:2481–2486. https://doi.org/10.1109/TBME.2008.923159
Zhang H, Guan L, Hai Y, Liu Y, Ding H, Chen X (2020) Multi-shot echo-planar diffusion tensor imaging in cervical spondylotic myelopathy. Bone Joint J 102-B:1210–1218. https://doi.org/10.1302/0301-620X.102B9.BJJ-2020-0468.R1
Wang K, Chen Z, Zhang F, Song Q, Hou C, Tang Y, Wang J, Chen S, Bian Y, Hao Q et al (2017) Evaluation of DTI parameter ratios and diffusion tensor tractography grading in the diagnosis and prognosis prediction of cervical spondylotic myelopathy. Spine (Phila Pa) 42:E202–E210. https://doi.org/10.1097/BRS.0000000000001784
Lope-Piedrafita S (2018) Diffusion Tensor Imaging (DTI). Methods Mol Biol. 1718:103–116. https://doi.org/10.1007/978-1-4939-7531-0_7
Basser PJ, Mattiello J, LeBihan D (1994) MR Diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. https://doi.org/10.1016/S0006-3495(94)80775-1
Hansen B (2019) An introduction to kurtosis fractional anisotropy. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A6235
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A (2018) Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front Neurol 9. https://doi.org/10.3389/fneur.2018.00092
Klawiter EC, Schmidt RE, Trinkaus K, Liang H-F, Budde MD, Naismith RT, Song S-K, Cross AH, Benzinger TL (2011) Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55:1454–1460. https://doi.org/10.1016/j.neuroimage.2011.01.007
Yousaf T, Dervenoulas G, Politis M (2018) Advances in MRI Methodology. Int Rev Neurobiol. 141:31–76. https://doi.org/10.1016/bs.irn.2018.08.008
Sharoh D, van Mourik T, Bains LJ, Segaert K, Weber K, Hagoort P, Norris DG (2019) Laminar Specific FMRI reveals directed interactions in distributed networks during language processing. Proc Natl Acad Sci 116:21185–21190.https://doi.org/10.1073/pnas.1907858116
Logothetis NK (2008) What we can do and what we cannot do with FMRI. Nature 453:869–878. https://doi.org/10.1038/nature06976
Lüdemann L, Förschler A, Wust P, Zimmer C (2007) Quantification of FMRI BOLD signal and volume applied to the somatosensory cortex. Z Med Phys 17:108–117. https://doi.org/10.1016/j.zemedi.2006.11.008
Klomjai W, Katz R, Lackmy-Vallée A (2015) Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (RTMS). Ann Phys Rehabil Med 58:208–213. https://doi.org/10.1016/j.rehab.2015.05.005
Jung SH, Shin JE, Jeong Y-S, Shin H-I (2008) Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol 119:71–79. https://doi.org/10.1016/j.clinph.2007.09.124
Hu M, Zeng N, Gu Z, Zheng Y, Xu K, Xue L, Leng L, Lu X, Shen Y, Huang J (2021) Short-term high-intensity interval exercise promotes motor cortex plasticity and executive function in sedentary females. Front Hum Neurosci 15. https://doi.org/10.3389/fnhum.2021.620958
Stefan K (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123:572–584. https://doi.org/10.1093/brain/123.3.572
Holly LT, Dong Y, Albistegui-Dubois R, Marehbian J, Dobkin B (2007) Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine 6:544–551. https://doi.org/10.3171/spi.2007.6.6.5
Tam S, Barry RL, Bartha R, Duggal N (2010) Changes in functional magnetic resonance imaging cortical activation after decompression of cervical spondylosis: Case Report. Neurosurgery 67. https://doi.org/10.1227/01.NEU.0000374848.86299.17
Nakamura M, Fujiyoshi K, Tsuji O, Konomi T, Hosogane N, Watanabe K, Tsuji T, Ishii K, Momoshima S, Toyama Y et al (2012) Clinical significance of diffusion tensor tractography as a predictor of functional recovery after laminoplasty in patients with cervical compressive myelopathy. J Neurosurg Spine 17:147–152. https://doi.org/10.3171/2012.5.SPINE1196
Bhagavatula ID, Shukla D, Sadashiva N, Saligoudar P, Prasad C, Bhat DI (2016) Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg Focus 40. https://doi.org/10.3171/2016.3.FOCUS1635
Costanzo R, Brunasso L, Paolini F, Benigno UE, Porzio M, Giammalva GR, Gerardi RM, Umana GE, di Bonaventura R, Sturiale CL et al (2022) Spinal tractography as a potential prognostic tool in spinal cord injury: a systematic review. World Neurosurg 164:25–32. https://doi.org/10.1016/j.wneu.2022.04.103
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SET, PRISMA, et al (2020) statement: an updated guideline for reporting systematic reviews. BMJ 2021:372. https://doi.org/10.1136/bmj.n71
Peng X, Tan Y, He L, Ou Y (2020) Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: a resting-state functional MRI study. NeuroReport 31:365–371. https://doi.org/10.1097/WNR.0000000000001346
Duggal N, Rabin D, Bartha R, Barry RL, Gati JS, Kowalczyk I, Fink M (2010) Brain reorganization in patients with spinal cord compression evaluated using FMRI. Neurology 74:1048–1054. https://doi.org/10.1212/WNL.0b013e3181d6b0ea
Sawada M, Nakae T, Munemitsu T, Hojo M (2018) Cortical reorganizations for recovery from depressive state after spinal decompression surgery. World Neurosurg 112:e632–e639. https://doi.org/10.1016/j.wneu.2018.01.108
Aleksanderek I, Stevens TK, Goncalves S, Bartha R, Duggal N (2017) Metabolite and functional profile of patients with cervical spondylotic myelopathy. J Neurosurg Spine 26:547–553. https://doi.org/10.3171/2016.9.SPINE151507
Ryan K, Goncalves S, Bartha R, Duggal N (2018) Motor network recovery in patients with chronic spinal cord compression: a longitudinal study following decompression surgery. J Neurosurg Spine 28:379–388. https://doi.org/10.3171/2017.7.SPINE1768
Green A, Cheong PWT, Fook-Chong S, Tiruchelvarayan R, Guo CM, Yue WM, Chen J, Lo YL (2015) Cortical reorganization is associated with surgical decompression of cervical spondylotic myelopathy. Neural Plast 2015. https://doi.org/10.1155/2015/389531
Paliwal M, Weber KA, Hopkins BS, Cantrell DR, Hoggarth MA, Elliott JM, Dahdaleh NS, Mackey S, Parrish TD, Dhaher Y et al (2020) Magnetization transfer ratio and morphometrics of the spinal cord associates with surgical recovery in patients with degenerative cervical myelopathy. World Neurosurg 144:e939–e947. https://doi.org/10.1016/j.wneu.2020.09.148
Lee JW, Kim JH, Bin Park J, Park KW, Yeom JS, Lee GY, Kang HS (2011) Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol 40:1543–1551. https://doi.org/10.1007/s00256-011-1161-z
Bhosale S, Ingale P, Srivastava S, Marathe N, Bhide P (2019) Diffusion tensor imaging as an additional postoperative prognostic predictor factor in cervical myelopathy patients: an observational study. J Craniovertebr Junction Spine 10:10–13. https://doi.org/10.4103/jcvjs.JCVJS_77_18
Jannelli G, Nouri A, Molliqaj G, Grasso G, Tessitore E (2020) Degenerative cervical myelopathy: review of surgical outcome predictors and need for multimodal approach. World Neurosurg 140:541–547. https://doi.org/10.1016/j.wneu.2020.04.233
Wang C, Ellingson BM, Islam S, Laiwalla A, Salamon N, Holly LT (2021) Supraspinal functional and structural plasticity in patients undergoing surgery for degenerative cervical myelopathy. J Neurosurg Spine 35:185–193. https://doi.org/10.3171/2020.11.SPINE201688
Wang C, Holly LT, Oughourlian T, Yao J, Raymond C, Salamon N, Ellingson BM (2021) Detection of cerebral reorganization associated with degenerative cervical myelopathy using diffusion spectral imaging (DSI). J Clin Neurosci 86:164–173. https://doi.org/10.1016/j.jocn.2021.01.011
Chen Q, Zheng W, Chen X, Li X, Wang L, Qin W, Li K, Chen N (2019) Reorganization of the somatosensory pathway after subacute incomplete cervical cord injury. Neuroimage Clin 21. https://doi.org/10.1016/j.nicl.2019.101674
Liu M, Tan Y, Zhang C, He L (2021) Cortical anatomy plasticity in cases of cervical spondylotic myelopathy associated with decompression surgery a strobe-compliant study of structural magnetic resonance imaging. Medicine (United States) 100. https://doi.org/10.1097/MD.0000000000024190
Wakabayashi T, Hidaka R, Fujimaki S, Asashima M, Kuwabara T (2014) MicroRNAs and epigenetics in adult neurogenesis. Adv Genet. 86:27–44. https://doi.org/10.1016/B978-0-12-800222-3.00002-4
Amtul Z (2015) Atta-Ur-Rahman neural plasticity and memory: molecular mechanism. Rev Neurosci 26:253–268. https://doi.org/10.1515/revneuro-2014-0075
Duffau H (2018) The error of Broca: from the traditional localizationist concept to a connectomal anatomy of human brain. J Chem Neuroanat 89:73–81
Duffau H (2021) The death of localizationism: the concepts of functional connectome and neuroplasticity deciphered by awake mapping, and their implications for best care of brain-damaged patients. Rev Neurol (Paris) 177:1093–1103
von Bernhardi R, Eugenín-Von Bernhardi L, Eugenín J (2017) What is neural plasticity? In Advances in Experimental Medicine and Biology; Springer New York LLC 1015:1–15
Kitano H (2007) Towards a theory of biological robustness. Mol Syst Biol 3. https://doi.org/10.1038/msb4100179
Gershenson C (2012) Guiding the self-organization of random Boolean networks. Theory Biosci 131:181–191. https://doi.org/10.1007/s12064-011-0144-x
Fauth M, Tetzlaff C (2016) Opposing effects of neuronal activity on structural plasticity. Front Neuroanat 10. https://doi.org/10.3389/fnana.2016.00075
Lisman J (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Phil Trans R Soc B: Biol Sci 372. https://doi.org/10.1098/rstb.2016.0260
Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM (2008) Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol 209:407. https://doi.org/10.1016/J.EXPNEUROL.2007.06.014
Zdunczyk A, Schwarzer V, Mikhailov M, Bagley B, Rosenstock T, Picht T, Vajkoczy P (2018) The corticospinal reserve capacity: reorganization of motor area and excitability as a novel pathophysiological concept in cervical myelopathy. Neurosurgery 83:810–818. https://doi.org/10.1093/NEUROS/NYX437
Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21:426–433. https://doi.org/10.1007/S00330-010-1927-Z
Jeurissen B, Leemans A, Tournier J-D, Jones DK, Sijbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766. https://doi.org/10.1002/hbm.22099
Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P (2008) Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage 42:617–625. https://doi.org/10.1016/j.neuroimage.2008.05.002
Smith RE, Tournier J-D, Calamante F, Connelly ASIFT (2013) Spherical-deconvolution informed filtering of tractograms. Neuroimage 67:298–312. https://doi.org/10.1016/j.neuroimage.2012.11.049
Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J (2014) Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103:411–426. https://doi.org/10.1016/j.neuroimage.2014.07.061
Holly LT, Ellingson BM, Salamon N (2017) Metabolic imaging using proton magnetic spectroscopy as a predictor of outcome after surgery for cervical spondylotic myelopathy. Clin Spine Surg 30:E615–E619. https://doi.org/10.1097/BSD.0000000000000248
Ellingson BM, Salamon N, Hardy AJ, Holly LT (2015) Prediction of neurological impairment in cervical spondylotic myelopathy using a combination of diffusion MRI and proton MR spectroscopy. PLoS One 10. https://doi.org/10.1371/JOURNAL.PONE.0139451
Zhao G, Zhang C, Zhan Y, He L (2022) The correlation between functional connectivity of the primary somatosensory cortex and cervical spinal cord microstructural injury in patients with cervical spondylotic myelopathy. Dis Markers 2022. https://doi.org/10.1155/2022/2623179
Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653. https://doi.org/10.1093/brain/awl359
Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41:1267–1277. https://doi.org/10.1016/j.neuroimage.2008.03.036
Zhang H, He W-J, Liang L-H, Zhang H-W, Zhang X-J, Zeng L, Luo S-P, Lin F, Lei Y (2021) Diffusion spectrum imaging of corticospinal tracts in idiopathic normal pressure hydrocephalus. Front Neurol 12. https://doi.org/10.3389/fneur.2021.636518
Wang C, Laiwalla A, Salamon N, Ellingson BM, Holly LT (2020) Compensatory brainstem functional and structural connectivity in patients with degenerative cervical myelopathy by probabilistic tractography and functional MRI. Brain Res 1749. https://doi.org/10.1016/J.BRAINRES.2020.147129
Chen Q, Zheng W, Chen X, Li X, Wang L, Qin W, Li K, Chen N (2019) Reorganization of the somatosensory pathway after subacute incomplete cervical cord injury. Neuroimage Clin 21:101674. https://doi.org/10.1016/J.NICL.2019.101674
Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH (2010) Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir (Wien) 152:1687–1694. https://doi.org/10.1007/s00701-010-0692-8
Shin J-W, Jin S-W, Kim S-H, Choi J-I, Kim B-J, Kim S-D, Lim D-J (2015) Predictors of Outcome in patients with cervical spondylotic myelopathy undergoing unilateral open-door laminoplasty. Korean J Spine 12:261. https://doi.org/10.14245/kjs.2015.12.4.261
Ghogawala Z, Terrin N, Dunbar MR, Breeze JL, Freund KM, Kanter AS, Mummaneni PV, Bisson EF, Barker FG, Schwartz JS et al (2021) Effect of ventral vs dorsal spinal surgery on patient-reported physical functioning in patients with cervical spondylotic myelopathy. JAMA 325:942. https://doi.org/10.1001/jama.2021.1233
Takenaka S, Kan S, Seymour B, Makino T, Sakai Y, Kushioka J, Tanaka H, Watanabe Y, Shibata M, Yoshikawa H et al (2020) Resting-state amplitude of low-frequency fluctuation is a potentially useful prognostic functional biomarker in cervical myelopathy. Clin Orthop Relat Res 478:1667–1680. https://doi.org/10.1097/CORR.0000000000001157
Zhou Y, Shi J (2022) Brain structural and functional dissociated patterns in degenerative cervical myelopathy: a case-controlled retrospective resting-state FMRI study. Front Neurol 13. https://doi.org/10.3389/fneur.2022.895348
Chen Z, Zhao R, Wang Q, Yu C, Li F, Liang M, Zong Y, Zhao Y, Xiong W, Su Z et al (2020) Functional connectivity changes of the visual cortex in the cervical spondylotic myelopathy patients: a resting-state FMRI study. Spine (Phila Pa 1976) 45:E272–E279. https://doi.org/10.1097/BRS.0000000000003245
Takenaka S, Kan S, Seymour B, Makino T, Sakai Y, Kushioka J, Tanaka H, Watanabe Y, Shibata M, Yoshikawa H, et al (2019) Towards prognostic functional brain biomarkers for cervical myelopathy: a resting-state FMRI study. Sci Rep 9. https://doi.org/10.1038/s41598-019-46859-5
Su Q, Zhao R, Wang SW, Tu HY, Guo X, Yang F (2021) Identification and therapeutic outcome prediction of cervical spondylotic myelopathy based on the functional connectivity from resting-state functional MRI data: a preliminary machine learning study. Front Neurol 12. https://doi.org/10.3389/fneur.2021.711880
Fan N, Zhao B, Liu LY, Yang WZ, Chen X, Lu ZB (2022) Dynamic and static amplitude of low-frequency fluctuation is a potential biomarker for predicting prognosis of degenerative cervical myelopathy patients: a preliminary resting-state FMRI study. Front Neurol 13. https://doi.org/10.3389/fneur.2022.829714
Zhao R, Guo X, Wang Y, Song YC, Su Q, Sun HR, Liang M, Xue Y (2022) Functional MRI evidence for primary motor cortex plasticity contributes to the disease’s severity and prognosis of cervical spondylotic myelopathy patients. Eur Radiol 32:3693–3704. https://doi.org/10.1007/S00330-021-08488-3
Craciunas SC, Gorgan MR, Ianosi B, Lee P, Burris J, Cirstea CM (2017) Remote motor system metabolic profile and surgery outcome in cervical spondylotic myelopathy. J Neurosurg Spine 26:668–678. https://doi.org/10.3171/2016.10.SPINE16479
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lapo Bonosi; methodology: Massimiliano Porzio and Luigi Cusimano; validation: Lapo Bonosi; formal analysis: Massimiliano Porzio; investigation: Massimiliano Porzio, Luigi Cusimano, Evier Andrea Giovannini and Umberto Emanuele Benigno; data curation: Lapo Bonosi and Massimiliano Porzio; writing—original draft preparation: Sofia Musso, Evier Andrea Giovannini, Umberto Emanuele Benigno, Massimiliano Porzio; figure/table preparation: Evier Andrea Giovannini writing—review and editing: Lapo Bonosi and Sofia Musso; visualization: Benedetta Campisi, Lara Brunasso, Roberta Costanzo, Federica Paolini, Kevin Giardina, Gianluca Scalia, Giuseppe Roberto Giammalva, Rosamaria Gerardi, and Rosario Maugeri; supervision: Lapo Bonosi and Rosario Maugeri; project administration: Lapo Bonosi, Rosario Maugeri, and Domenico Gerardo Iacopino. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is a systematic review, and no ethical approval is required.
Informed consent statement
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonosi, L., Musso, S., Cusimano, L.M. et al. The role of neuronal plasticity in cervical spondylotic myelopathy surgery: functional assessment and prognostic implication. Neurosurg Rev 46, 149 (2023). https://doi.org/10.1007/s10143-023-02062-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02062-9