Abstract
To perform a systematic review of extracranial-to-intracranial (EC-IC) bypass surgery with parent vessel trapping for blood blister–like aneurysms (BBAs) of the internal carotid artery (ICA) according to PRISMA guidelines. Search of PubMed using “bypass” [all fields] and “ICA” [all fields] or “internal carotid artery” [all fields] and (“blood blister–like aneurysm” [MeSH terms]. Thirty-four original articles were identified, of which 21 were excluded (treatment not including bypass or insufficient details on complications or clinical outcomes). Thirteen articles published between 2008 and 2019 were included, totaling 98 patients, with a median of 7.5 patients per article (range 1–17). Mean age was 53.3 years (range 23–80). The main techniques were external carotid artery to middle cerebral artery (ECA-MCA) in 81% and superficial temporal artery to MCA (STA-MCA) in 19%. The most common grafts were radial artery (74%) and STA (19%). The risk of intraoperative rupture varied from 0 to 75%, with a mean of 12%. With respect to clinical outcomes, the modified Rankin Scale (mRS) was not stated in 30% of the cases. When stated, mRS was ≤ 2 in 79%, mRS was 3–5 in 10%, and 4% had mRS 6 (death). We identified only 13 articles, with no prospective studies. Outcomes were better than generally reported for ruptured aneurysms, both with respect to poor outcome (mRS > 2) and in-hospital mortality, perhaps reflecting a selection bias. In general, the data reporting quality was low, precluding any firm conclusions, but EC-IC bypass with ICA trapping may be a valid treatment option for ruptured ICA BBAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite being rare, representing only 0.9–6.5% of all internal carotid artery (ICA) aneurysms, 1% of all intracranial aneurysms, and 0.5–2% of all ruptured intracranial aneurysms [1,2,3], blood blister–like aneurysms (BBAs) are challenging by nature of their fragility, small size, and clinical presentation. BBAs can occur in any intracranial artery but are more frequent in the supraclinoid ICA and then typically arise from the anterior wall [1, 3]. In contrast to saccular or berry-type aneurysms, BBAs are thought to emerge as a result of hemodynamic stress, atherosclerosis, or dissection [4,5,6], and their walls are composed of a thin layer of adventitia covering a focal defect in the intima and media and are located at non-branching sites of the cerebral circulation [3, 5, 7, 8].
Numerous treatments have been reported to overcome these specific and unique characteristics of BBAs [9]. Endovascular techniques include balloon-assisted coiling [10], stent-assisted coiling [11], covered stenting [11, 12], overlapping stents [13,14,15], flow diverters [16, 17], and parent vessel occlusion [3, 9]. There are different microsurgical options such as direct clipping [3, 9, 18, 19], clip wrapping [20, 21], trapping with or without an extracranial-to-intracranial bypass [19, 22,23,24,25,26,27,28,29,30,31], and primary suturing [19, 32, 33]. These techniques all carry various advantages and drawbacks [9]. Whereas the necessity of dual antiplatelet therapy may preclude endovascular therapy, clip reconstruction may fail either due to intraoperative aneurysm rupture, parent vessel stenosis, or focal vessel avulsion [3, 34].
Based on a cadaveric study, Ishikawa et al. [35] found that many BBAs are focal wall defects covered with thin fibrous tissue and that they are not true aneurysms. It was also found that many of them had an arterial dissection as their pathogenesis, making trapping of the ICA segment with the BBA the most effective surgical option [30, 36]. However, in the acute phase, ICA trapping leads to poor outcomes with a high risk of cerebral ischemia due to vasospasm and insufficient blood flow to the contralateral side despite a positive BTO [3].
Their friable nature means that BBAs are at risk of intraoperative rupture when direct clipping is attempted and intraoperative bleeding is frequently fatal [3, 37, 38]. In 2008, Meling et al. [3] demonstrated that ICA sacrifice in the acute stage of SAH inadvertently results in poor outcomes in patients not treated with combined revascularization surgery, and they hypothesized that high-flow extracrania- to-intracranial (EC-IC) bypass, when performed within the time-frame prior to the onset of vasospasms, may prevent vasospasm-induced cerebral infarcts and subsequent deaths associated with acute ICA sacrifice in SAH. Furthermore, an EC-IC bypass may lower the risk of intraoperative rupture since there is no dissection of the BBA [39]. Later, Kazumata et al. [27] demonstrated the proof of concept of EC-IC bypass in 20 patients with ICA BBAs by using RA graft bypass with parent vessel sacrifice during the acute phase of SAH. Since then, the use of an EC-IC bypass from the external carotid artery (ECA) or the superficial temporal artery (STA) to the middle cerebral artery (MCA) has emerged as valuable adjuncts. The technique particularly used for flow replacement when ICA sacrifice occurs [28, 40] or as a protective bypass prior to exploration and intended clipping of the ICA BBA [27].
The aim of this systematic review was to analyze the advantages and drawbacks IC-EC bypass in the treatment of ICA BBAs.
Material and methods
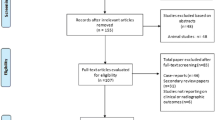
A search of PubMed databases for the last 11 years (from 2008 to 2019) using the following criteria, “bypass” [all fields] and “ICA” [all fields] or “internal carotid artery” [all fields] and (“blood blister–like aneurysm” [MeSH terms], was performed. Basic inclusion filters were English language and articles providing information on the type of bypass used, complications, and clinical outcomes. Articles not related to the use of bypass for the treatment of ICA BBA were excluded. A PRISMA flow diagram was created in order to analyze the recent literature (Fig. 1) [41].
Results
The literature search identified 34 articles, 21 of which were excluded due to treatment not including a bypass, not being in English, or not giving detailed information about the complications or the clinical outcomes (Fig. 1). The final 13 articles were compared with respect to number of patients, patient age, gender, BBA localization, rupture status, preoperative clinical scores, intention of the bypass, type of bypass used, intraoperative rupture rates, complications, and postoperative clinical outcomes (Tables 1 and 2).
In summary, the analysis showed that (Table 3):
-
1.
The total number of patients was 98, with a median of 7.5 patients per article included in this review (range 1–17).
-
2.
The patients’ age was 53.3 years (range 23–80).
-
3.
There was a predominance of females with 62% vs. 38% males.
-
4.
The anterior, medial, or anteromedial wall of ICA was the most common location of the BBA with 56% compared with 24% of the posterior wall and 16% on the lateral wall. The localization was not stated in 37% of the cases (Table 3).
-
5.
With respect to laterality, this information was not available in 63% of the cases (Table 3). The BBAs were located on the right side in 58% when stated.
-
6.
All the cases were ruptured when explicitly stated, but information was lacking for 39 patients (40%) (Table 3). Likewise, the preoperative Fisher grade was not stated in 42%.
-
7.
With respect to the preoperative clinical condition, a Hunt and Hess score or a WFNS score was not stated in 67 and 33% of the cases, respectively. When reported, the clinical scores varied widely between individuals, from pauci- or asymptomatic (WFNS score < 3) in 56% to poor neurological condition (WFNS score ≥ 3) to) in 44% (Table 3).
-
8.
The intention of the bypass was pre hoc in 84%, post hoc in 2%, and not stated in 14%. The intention of pre hoc bypasses were either protective (followed by aneurysm clipping) or replacement (followed by ICA trapping) (Table 2).
-
9.
The main techniques used for IC-EC bypass were ECA-MCA (81%) and STA-MCA (19%). The most common grafts were radial artery (RA) (74%) and superficial temporal artery (STA) (19%), while the saphenous vein (SA) was less commonly used (7%) (Table 3).
-
10.
The risk of intraoperative rupture varied according to the different series. Indeed, some authors had a 75% intraoperative rupture rate, while others reported none. Overall, the mean intraoperative rupture was 12%.
-
11.
In terms of complications, 10% of the patients developed vasospasms, and 23% had infarcts or contusions (Table 3).
-
12.
With respect to clinical outcomes, the modified Rankin Scale (mRS) was not stated in 30% of the cases. When stated, the outcome was good (mRS score ≤ 2) in 79%, whereas 10% had moderate to severe disabilities (mRS 3–5) and 4% had mRS 6 (death). When using the Glasgow Outcome Scale, good recovery was seen in 71%, mild disability in 10%, severe disability in 13%, and death in 4% (Table 3).
Discussion
This systematic review analyzed the use of IC-EC bypass in the treatment of ICA BBAs. We identified only 13 articles (Fig. 1, Tables 1 and 2), and in general, the quality of the data reporting was low (Table 3). There were no prospective studies identified. In the papers included, the total number of patients was 98, with a mean of 7.5 patients per article (range 1–17), reflecting the small population affected by ICA BBAs and inferring that statistical meta-analysis would be futile.
BBAs are more frequently seen at a younger age [3, 7, 20, 45] and occur more frequently in females [3, 20, 46, 47] and on the right ICA [3, 7, 20, 47, 48] than the typical saccular ICA aneurysms. In the papers reviewed, mean patient age was 53.3 years, and 62% of the patients were females (Table 1). The BBAs were located on the right side in 58% of cases, and the anterior or anteromedial wall of ICA was the most common location of the BBA with 56%. This is in line with previous reports [3, 7, 20].
The majority of the cases were ruptured, but in 40% of the cases, the rupture status was not stated (Table 1). This is in line with previous reports where the vast majority of BBAs present with SAH [3, 7, 20, 39, 49]. With respect to preoperative clinical condition, neither a Hunt and Hess score nor a WFNS score was stated in 67 and 33% of cases, respectively. When stated, the clinical presentation varied widely between individuals, from a- or pauci-symptomatic (WFNS score < 3) in 56% to poor neurological condition (WFNS score ≥ 3) to) in 44%, which is in line with the scientific literature on aneurysmal SAH in general [50].
With respect to the intention of EC-IC bypass, it can be used pre hoc or up-front when ICA trapping is planned or when a primary clip reconstruction was planned preoperatively, but the intraoperative inspection suggests an excessive friability of the BBA [18, 27, 28, 35, 43]. Despite requiring more time and entails increased complexity, this strategy reduces the risk of a devastating intraoperative hemorrhage [18, 35, 39]. In our review, the intention of the bypass was pre hoc in 84%, post hoc in 2%, and unknown in 14%. A pre hoc intention was either protective (followed by aneurysm clipping) or replacement (followed by ICA trapping).
The bypass graft types were RA (74%), STA (19%), and SV (7%). The choice of bypass graft might be based on surgeon preference or estimations of collateral flow, as assessed by a preoperative angiography [51]. However, although donor graft selection remains debated [52], a RA graft is often preferred [27, 40]. Arteries are valve-free and may remain open even at low flow rates, qualities that might contribute to superior long-term patency rates of RA grafts [44]. Furthermore, RA and STA grafts are less vulnerable to traumatic injury during harvesting, and arterial grafts are generally more resistant to kinking and torsion than venous grafts. However, a SV graft has the advantages of its long length, easy manipulability, and high-flow conduit [53], but the discrepancy in diameter between a SV donor graft and a MCA recipient vessel is a significant limitation as it may create turbulent flow at the anastomosis site and cause delayed graft occlusion [44]. In this review, all graft types had good patency rates, and there were no significant differences between them in terms of outcomes (Table 3). Ischemic stroke was seen in 21% of the patients (Table 3).
Severe symptomatic vasospasms may develop in SAH patients [34, 50], and if it occurs in the territory of a major cerebral artery, cerebral angioplasty, selective intra-arterial vasodilator therapy, or both can be considered [54]. The bypass conduit can potentially be used as a route of endovascular drug administration to treat vasospasm when it is necessary [35]. STA-MCA bypasses allow for the use of intra-arterial vasodilators, whereas RA grafts can even be used for super-selective intra-arterial drug instillations, and large conduits like the SV grafts may even allow for catheter angioplasty via the graft. Furthermore, considering the risk of vasospasm and the deleterious effects of such in the acute stage of a SAH [3], a high-flow ECA-MCA bypass with RA or SV donor grafts may be advantageous over a low-flow STA-MCA bypass in maintaining perfusion pressure and cerebral blood flow in the ICA after trapping [27, 40]. In this systematic review, high-flow bypass was used in 81% of the cases (Table 3).
In the papers reviewed, the risk of intraoperative aneurysm rupture varied between 0 and 75% (Table 2). Overall, the mean intraoperative rupture rate was 12% (Table 3), which is much lower than that associated with direct microsurgical clipping that typically carries a 30–50% risk of intraoperative rupture [3, 7, 18, 33, 46, 55,56,57,58,59,60] This indicates a significant risk-reducing effect of an EC-IC bypass in ruptured ICA BBAs [27, 39], and an intraoperative rupture rate of 12% is on par with the clip-wrap technique, albeit in small case series [19, 20, 48, 56, 61].
With respect to clinical outcomes, the data quality was generally very poor as the modified Rankin Scale (mRS) was not stated in 30% of the cases (Table 3). When stated, the outcome was good (mRS score ≤ 2) in 79%, whereas 10% had moderate to severe disabilities (mRS 3–5), and 4% had mRS 6 (death). When the Glasgow Outcome Scale was used, good recovery was seen in 71%, mild disability in 10%, severe disability in 13%, and death in 4% (Table 3). These results are better than generally reported for acute SAH, both with respect to poor outcome (mRS > 2) and the in-hospital mortality [62, 63], perhaps reflecting a selection bias.
Although EC-IC bypass with ICA trapping may be a valid treatment option for ruptured ICA BBAs, many clinical series have employed various surgical and endovascular techniques, reflecting a lack of solid evidence of superiority of any method [3, 9, 20, 42, 58]. Whereas microsurgery offers superior obliteration rates and neurological outcomes on par with endovascular treatments [9], it comes at the price of a higher complication rates. In contrast, endovascular therapy offers superior safety and provides functional outcomes comparable with surgery [9, 46, 57], albeit at a higher financial cost both in terms of retreatments, regular angiographic controls, and device costs. Multilayer flow-diverting stents appear to be a promising strategy, but at present, flow diversion devices (FDDs) lack sufficient evidence from trials and have only been used for a short period of time [9, 46], and the double antiplatelet therapy is still a major constraint in the setting of SAH.
Conclusions
In this systematic review, we identified only 13 articles, with no prospective studies. Outcomes were better than generally reported for ruptured aneurysms, both with respect to poor outcome (mRS > 2) and in-hospital mortality [62, 63], perhaps reflecting a selection bias. Furthermore, in general, the data reporting quality was low, precluding any firm conclusions, but EC-IC bypass with ICA trapping may be a valid treatment option for ruptured ICA BBAs.
References
Abe M, Tabuchi K, Yokoyama H, Uchino A (1998) Blood blister-like aneurysms of the internal carotid artery. J Neurosurg 89(3):419–424
Chalouhi N, Zanaty M, Tjoumakaris S, Gonzalez LF, Hasan D, Kung D et al (2014) Treatment of blister-like aneurysms with the pipeline embolization device. Neurosurgery. 74(5):527–532 discussion 32
Meling TR, Sorteberg A, Bakke SJ, Slettebo H, Hernesniemi J, Sorteberg W (2008) Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg 108(4):662–671
Mitha AP, Spetzler RF (2011) Blister-like aneurysms: an enigma of cerebrovascular surgery. World Neurosurg 74(4–5):444–445
Sugar O (1992) It's remarkable that there should be a carotid-ophthalmic aneurysm that is anterior or dorsal in the proximal internal carotid artery, and hence unrelated to any arterial junction. Surg Neurol 37(2):158
Diraz A, Kyoshima K, Kobayashi S (1993) Dorsal internal carotid artery aneurysm: classification, pathogenesis, and surgical considerations. Neurosurg Rev 16(3):197–204
Sim SY, Shin YS, Cho KG, Kim SY, Kim SH, Ahn YH, Yoon SH, Cho KH (2006) Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg 105(3):400–405
Ogawa A, Suzuki M, Ogasawara K (2000) Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 47(3):578–583 discussion 83-6
Meling TR (2017) What are the treatment options for blister-like aneurysms? Neurosurg Rev 40(4):587–593
Park JH, Park IS, Han DH, Kim SH, Oh CW, Kim JE et al (2007) Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg 106(5):812–819
Lee BH, Kim BM, Park MS, Park SI, Chung EC, Suh SH, Choi CS, Won YS, Yu IK (2009) Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg 110(3):431–436
Fang C, Tan HQ, Han HJ, Feng H, Xu JC, Yan S et al (2017) Endovascular isolation of intracranial blood blister-like aneurysms with Willis covered stent. J Neurointerv Surg 9(10):963–968. https://doi.org/10.1136/neurintsurg-2016-012662
Song J, Oh S, Kim MJ, Chung J, Lim YC, Kim BS, Shin YS (2016) Endovascular treatment of ruptured blood blister-like aneurysms with multiple (>/=3) overlapping Enterprise stents and coiling. Acta Neurochir 158(4):803–809
Walsh KM, Moskowitz SI, Hui FK, Spiotta AM (2013) Multiple overlapping stents as monotherapy in the treatment of 'blister' pseudoaneurysms arising from the supraclinoid internal carotid artery: a single institution series and review of the literature. J Neurointerv Surg 6(3):184–194
Fiorella D, Albuquerque FC, Deshmukh VR, Woo HH, Rasmussen PA, Masaryk TJ et al (2006) Endovascular reconstruction with the neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 59(2):291–300 discussion 291-300
Aydin K, Arat A, Sencer S, Hakyemez B, Barburoglu M, Sencer A et al (2014) Treatment of ruptured blood blister-like aneurysms with flow diverter SILK stents. J Neurointerv Surg 7(3):202–209
Heiferman DM, Billingsley JT, Kasliwal MK, Johnson AK, Keigher KM, Frudit ME, Moftakhar R, Lopes DK (2015) Use of flow-diverting stents as salvage treatment following failed stent-assisted embolization of intracranial aneurysms. J Neurointerv Surg 8(7):692–695
Owen CM, Montemurro N, Lawton MT (2017) Blister aneurysms of the internal carotid artery: microsurgical results and management strategy. Neurosurgery. 80(2):235–247
Lee JW, Choi HG, Jung JY, Huh SK, Lee KC (2009) Surgical strategies for ruptured blister-like aneurysms arising from the internal carotid artery: a clinical analysis of 18 consecutive patients. Acta Neurochir 151(2):125–130
Meling TR, Patet G (2019) Clip-wrapping of ruptured blood blister-like aneurysms of the internal carotid artery. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01172-7
Safavi-Abbasi S, Moron F, Sun H, Wilson C, Frock B, Oppenlander ME et al (2016) Techniques and outcomes of Gore-Tex clip-wrapping of ruptured and unruptured cerebral aneurysms. World Neurosurg 90:281–290
Aihara M, Shimizu T, Naito I, Miyamoto N, Yoshimoto Y (2019) Surgical strategies and clinical results of site-specific treatment using high-flow bypass for ruptured blood blister-like anterior wall aneurysms of the internal carotid artery. World Neurosurg 125:e1247–e1e55
Baskaya MK, Ahmed AS, Ates O, Niemann D (2008) Surgical treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery with extracranial-intracranial bypass and trapping. Neurosurg Focus 24(2):E13
Cikla U, Baggott C, Baskaya MK (2014) How I do it: treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery by extracranial-to-intracranial bypass and trapping. Acta Neurochir 156(11):2071–2077
Endo H, Fujimura M, Shimizu H, Endo T, Omodaka S, Inoue T, Sato K, Niizuma K, Tominaga T (2020) Optimal Timing of Extracranial-Intracranial Bypass with Microsurgical Trapping for Ruptured Blister Aneurysms of the Internal Carotid Artery. World Neurosurg 136:e567–e577
Kawashima A, Okada Y, Kawamata T, Onda H, Kubo O, Hori T (2008) Successful treatment of a blood blister-like aneurysm of the internal carotid artery by trapping with a high-flow bypass. J Clin Neurosci 15(7):797–800
Kazumata K, Nakayama N, Nakamura T, Kamiyama H, Terasaka S, Houkin K (2014) Changing treatment strategy from clipping to radial artery graft bypass and parent artery sacrifice in patients with ruptured blister-like internal carotid artery aneurysms. Neurosurgery. 10(Suppl 1):66–72 discussion 3
Kikkawa Y, Ikeda T, Takeda R, Nakajima H, Ogura T, Ooigawa H, Kurita H (2017) Results of early high-flow bypass and trapping for ruptured blood blister-like aneurysms of the internal carotid artery. World Neurosurg 105:470–477
Kim YS, Joo SP, Kim TS (2019) Microsurgical Management of ruptured blood blister aneurysms of the internal carotid artery without bypass: a retrospective single-center study of 36 patients over 20 years. World Neurosurg 128:e956–ee65
Kubo Y, Koji T, Yoshida K, Saito H, Ogawa A, Ogasawara K (2015) High-flow bypass and wrap-clipping for ruptured blood blister-like aneurysm of the internal carotid artery using intraoperative monitoring of cerebral hemodynamics. Vasc Health Risk Manag 11:297–302
Yang K, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ (2014) The efficacy of bypass surgery using a short interposition graft for the treatment of intracranial complex aneurysm. World Neurosurg 83(2):197–202
Chen F, Zhang L, Cheng Q, Huang Z, Huang J, Wang J (2018) Suturing treatment for blood blister-like aneurysm in supraclinoid segment of internal carotid artery. World Neurosurg 109:271–274
Joo SP, Kim TS, Moon KS, Kwak HJ, Lee JK, Kim JH et al (2006) Arterial suturing followed by clip reinforcement with circumferential wrapping for blister-like aneurysms of the internal carotid artery. Surg Neurol 66(4):424–428 discussion 8-9
Balik V, Takebayashi S, Takizawa K (2020) A case series of double bypass technique used for the treatment of internal carotid blood blister-like aneurysms in patients in poor initial neurological condition at the early stage of subarachnoid hemorrhage. Oper Neurosurg (Hagerstown) 18(2):126–135
Ishikawa T, Mutoh T, Nakayama N, Yasuda H, Nomura M, Kazumata K et al (2009) Universal external carotid artery to proximal middle cerebral artery bypass with interposed radial artery graft prior to approaching ruptured blood blister-like aneurysm of the internal carotid artery. Neurol Med Chir (Tokyo) 49(11):553–558
Murai Y, Mizunari T, Umeoka K, Tateyama K, Kobayashi S, Teramoto A (2012) Ischemic complications after radial artery grafting and aneurysmal trapping for ruptured internal carotid artery anterior wall aneurysm. World Neurosurg 77(1):166–171
Hongo K, Sato A, Kakizawa Y, Miyahara T, Tanaka Y, Sugiyama T (2006) The nationwide surveillance on the dorsal aneurysm of the internal carotid artery part 1: analysis of the factors affecting the poor outcome. Surg Cereb Stroke 34(5):366–371
Satoh A, Sugiyama T, Hongo K, Kakizawa Y, Ishihara S, Matsutani M (2006) The nationwide surveillance on the dorsal aneurysm of the internal carotid artery part 2: study on the surgical treatment in hemorrhagic cases. Surg Cereb Stroke (Jpn). 34(5):372–376
Strickland BA, Rennert RC, Bakhsheshian J, Ravina K, Fredrickson V, Giannotta SL et al (2018) Extracranial-intracranial bypass for treatment of blister aneurysms: efficacy and analysis of complications compared with alternative treatment strategies. World Neurosurg 117:e417–ee24
Kamijo K, Matsui T (2010) Acute extracranial-intracranial bypass using a radial artery graft along with trapping of a ruptured blood blister-like aneurysm of the internal carotid artery. Clinical article. J Neurosurg 113(4):781–785
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Gonzalez AM, Narata AP, Yilmaz H, Bijlenga P, Radovanovic I, Schaller K, Lovblad KO, Pereira VM (2014) Blood blister-like aneurysms: single center experience and systematic literature review. Eur J Radiol 83(1):197–205
Sorimachi T, Osada T, Hirayama A, Shigematsu H, Srivatanakul K, Matsumae M (2019) Preservation of anterior choroidal artery blood flow during trapping of the internal carotid artery for a ruptured blood blister-like aneurysm with high-flow bypass. World Neurosurg 122:e847–ee55
Yang K, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ (2015) The efficacy of bypass surgery using a short interposition graft for the treatment of intracranial complex aneurysm. World Neurosurg 83(2):197–202
Shigeta H, Kyoshima K, Nakagawa F, Kobayashi S (1992) Dorsal internal carotid artery aneurysms with special reference to angiographic presentation and surgical management. Acta Neurochir 119:42–48
Szmuda T, Sloniewski P, Waszak PM, Springer J, Szmuda M (2015) Towards a new treatment paradigm for ruptured blood blister-like aneurysms of the internal carotid artery? A rapid systematic review. J Neurointerv Surg 8(5):488–494
Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, Sumiyoshi K, Miyawaki H, Aoyagi C, Takeuchi S, Suzuki G (2009) Clinical and radiological findings and surgical management of ruptured aneurysms at the non-branching sites of the internal carotid artery. J Clin Neurosci 16(8):1018–1023
Nakagawa F, Kobayashi S, Nagamine Y, Yanagida N, Mikawa S, Suzuki A (1986) Aneurysms protruding from the dorsal wall of the internal carotid artery. J Neurosurg 65:303–308
Rouchaud A, Brinjikji W, Cloft HJ, Kallmes DF (2015) Endovascular treatment of ruptured blister-like aneurysms: a systematic review and meta-analysis with focus on deconstructive versus reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol 36(12):2331–2339
Lawton MT, Vates GE (2017) Subarachnoid hemorrhage. N Engl J Med 377(3):257–266
Endo H, Fujimura M, Shimizu H, Inoue T, Sato K, Niizuma K, Tominaga T (2015) Cerebral blood flow after acute bypass with parent artery trapping in patients with ruptured Supraclinoid internal carotid artery aneurysms. J Stroke Cerebrovasc Dis 24(10):2358–2368
Ramanathan D, Temkin N, Kim LJ, Ghodke B, Sekhar LN (2012) Cerebral bypasses for complex aneurysms and tumors: long-term results and graft management strategies. Neurosurgery. 70(6):1442–1457 discussion 57
Marhold F, Rosen CL (2010) Novel technique to improve vessel mismatch when using saphenous vein bypass grafts for intracranial revascularization procedures. J Neurosurg 112(6):1227–1231
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 43(6):1711–1737
Charbel FT, Gonzales-Portillo G, Hoffman W, Cochran E (1999) Distal internal carotid artery pseudoaneurysms: technique and pitfalls of surgical management: two technical case reports. Neurosurgery. 45(3):643–648 discussion 8-9
Ogawa A, Suzuki A, Ogasawara K (2000) Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid trunk aneurysms. Neurosurgery. 47:578–586
Shah SS, Gersey ZC, Nuh M, Ghonim HT, Elhammady MS, Peterson EC (2017) Microsurgical versus endovascular interventions for blood-blister aneurysms of the internal carotid artery: systematic review of literature and meta-analysis on safety and efficacy. J Neurosurg. 127(6):1361–1373.https://doi.org/10.3171/2016.9.JNS161526
Konczalla J, Gessler F, Bruder M, Berkefeld J, Marquardt G, Seifert V (2017) Outcome after subarachnoid hemorrhage from blood blister-like aneurysm rupture depends on age and aneurysm morphology. World Neurosurg 105:944–951.e1
Kalani MY, Zabramski JM, Kim LJ, Chowdhry SA, Mendes GA, Nakaji P, McDougall C, Albuquerque FC, Spetzler RF (2013) Long-term follow-up of blister aneurysms of the internal carotid artery. Neurosurgery. 73(6):1026–1033 discussion 33
Pahl FH, de Oliveira MF, Teles Gomes Mde Q, Capel Cardoso AC, Rotta JM (2016) Blister-like aneurysms: report of successful surgical treatment of consecutive cases and review of the literature. World Neurosurg 89:376–381
Safavi-Abbasi S, Moron F, Sun H, Oppenlander ME, Kalani MY, Mulholland CB, Zabramski JM, Nakaji P, Spetzler RF (2016) Techniques and long-term outcomes of cotton-clipping and cotton-augmentation strategies for management of cerebral aneurysms. J Neurosurg 125(3):720–729
Ikawa F, Michihata N, Matsushige T, Abiko M, Ishii D, Oshita J, Okazaki T, Sakamoto S, Kurogi R, Iihara K, Nishimura K, Morita A, Fushimi K, Yasunaga H, Kurisu K (2019) In-hospital mortality and poor outcome after surgical clipping and endovascular coiling for aneurysmal subarachnoid hemorrhage using nationwide databases: a systematic review and meta-analysis. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01096-2
Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, Commichau C, Connolly ES, Mayer SA (2005) Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 62(3):410–416
Funding
Open Access funding provided by University of Oslo (incl Oslo University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approved
Not applicable as no new patients were involved in this research.
Informed consent
Not applicable as no new patients were involved in this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meling, T.R., Patet, G. The role of EC-IC bypass in ICA blood blister aneurysms—a systematic review. Neurosurg Rev 44, 905–914 (2021). https://doi.org/10.1007/s10143-020-01302-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01302-6