Abstract
The lack of a simple, objective and reproducible system to describe glioblastoma multiforme (GBM) represents a major limitation in comparative effectiveness research. The objectives of this study were therefore to develop such a grading system and to validate it on patients who underwent surgical resection. A systematic review of the literature was performed to identify features on pre-operative magnetic resonance imaging (MRI) that predict the surgical outcome of patients with GBM. In all, the five most important features of GBM on pre-operative MRI were as follows: periventricular or deep location, corpus callosum or bilateral location, eloquent location, size and associated oedema. These were then used to develop a grading system. To validate this grading system, a retrospective cohort study of all adult patients with supratentorial GBM who underwent surgical resection between the 1 January 2014 and the 31 June 2015 was performed. There was a substantial agreement between the two neurosurgeons grading GBM (Cohen’s κ was 0.625; standard error 0.066). High-complexity lesions were significantly less likely to result in complete resection of contrast-enhancing tumour than low-complexity lesions (50.0 versus 3.4%; p = 0.0007). The proposed grading system may allow for the standardised communication of anatomical features of GBM identified on pre-operative MRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surgical management of patients with glioblastoma multiforme (GBM) remains contentious with a paucity of high-quality evidence to guide decision making. Nonetheless, the nihilism associated with GBM is gradually diminishing. A randomised study concluded that, even in the elderly, surgical resection was superior to biopsy alone [43]. More recently, cohort studies have found that complete resection of enhancing tumour results in prolonged survival [27, 29, 34, 39]. However, in the largest prospective study on patients with GBM, complete resection was only achieved in approximately a third of patients, suggesting difficulty in defining marginal, enhancing tumour intra-operatively using conventional microsurgical techniques [38].
A multitude of surgical innovations have been introduced to maximise the resection of GBM including fluorescence-guided surgery and various other intraoperative imaging techniques [3, 20, 26]. Over the next decade, emerging technologies such as confocal laser endomicroscopy, rapid evaporative ionisation mass spectrometry and Raman spectroscopy are expected to further expand the surgical armamentarium [2, 19, 24].
Selecting from the aforementioned array of surgical innovations is difficult. The operative techniques used for the resection of GBM have historically been based on a surgeon’s training and experience and the local availability of resources. The Balliol Collaboration has proposed the Idea, Development, Exploration, Assessment and Long-term follow up (IDEAL) model for surgical innovation; the central tenet being that innovation and evaluation should proceed together [13, 17, 31]. Evaluation of surgical innovations that maximise the resection of GBM is especially challenging because of the heterogeneity in tumour anatomy and the complexity of surgical resection.
In other cerebral pathologies such as arteriovenous malformation (AVM), several anatomical features on pre-operative imaging have been found to predict surgical outcome including size, relationship with eloquent structures and pattern of venous drainage [37]. The lack of a similarly simple, objective and reproducible system to describe GBM represents a major limitation in comparative effectiveness research. The aims of this study were therefore to (1) identify features on standard pre-operative magnetic resonance imaging (MRI) that predict the surgical outcome of patients with GBM and use these to develop a grading system and (2) validate this grading system on patients who have underwent surgical resection.
Materials and methods
Development
A systematic review of the literature was performed to identify features on pre-operative MRI that predict the surgical outcome of patients with GBM. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used in the preparation of this section of the manuscript [32].
Inclusion and exclusion criteria
Articles that (1) featured adult patients (greater than 18 years of age) with supratentorial GBM undergoing surgical resection and (2) reported on the use of pre-operative MRI to predict residual disease or survival were included. Articles that used advanced imaging techniques such as segmentation and volumetric analysis, which are not widely available, were excluded.
Information sources, search strategy and study selection
The PubMed database was searched between 1 January 1995 and 31 June 2015 using the search terms (glioblastoma OR “malignant glioma” OR “high grade glioma” OR “high-grade glioma”) AND (pre-operative OR preoperative OR preop OR pre-op) AND (prediction OR predictive OR scoring OR score) AND (outcome OR resection OR resectability OR “progression free survival” OR PFS OR “overall survival” OR OS).
Titles and abstracts were screened to identify articles that met appeared to meet the inclusion criteria (HJM and AHH; both clinical research fellows). Full papers were then retrieved for detailed review. Discrepancies were resolved by consensus and discussion with the senior author.
Data collection process and data items
The following data were extracted from selected articles (HJM and AHH): (1) study design, (2) patient characteristics, (3) pre-operative MRI features, (4) surgical outcomes and (5) findings. When both univariate and multivariate analyses were presented, we preferentially included the more significant findings. Corresponding authors were contacted to provide supplemental data when required.
Development of grading system
The pre-operative MRI features most frequently used to predict surgical outcomes were used to develop a grading system. Features were selected such that the proposed grading system made clinical sense and could be easily incorporated into practice using a standard contrast-enhanced T1-weighted MRI.
Validation
A retrospective cohort study was performed to validate the grading system. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was used in the preparation of this section of the manuscript [42].
Setting and participants
The study was conducted at Charing Cross Hospital, which acts as regional referral centre for brain tumours in North West London. The centre comprises three specialist neurosurgeons, who spend at least half of their clinical programmed activity in neurooncological surgery.
All referrals were recorded on a prospectively maintained database. The database was searched between the 1 January 2014 and the 31 June 2015 to identify all adult patients with supratentorial GBM who underwent craniotomy and resection. Patients who underwent a burr hole and stereotactic biopsy only were excluded.
Variables and data sources
All patients with GBM received therapy according to the National Institute of Clinical Excellence (NICE) guidance, including the following: high-dose dexamethasone, discussion of their case in a dedicated neurooncology multidisciplinary meeting, pre-operative MRI with contrast, image-guided craniotomy and microsurgical resection and post-operative MRI with contrast within 72 h of surgery. Intraoperative ultrasound was available but was used according to surgeon preference.
The pre-operative contrast-enhanced T1-weighted MRI scans were graded by two neurosurgeons blinded to the outcome. The post-operative contrast-enhanced T1-weighted MRI scan was evaluated by a consultant neuroradiologist blinded to the grade to determine the extent of resection (complete resection of all contrast-enhancing tumours or not). All images were reviewed on standard display monitors.
A retrospective case note review was also performed to identify any immediate surgical complications, which were recorded according the Clavian-Dindo classification [12, 15]. The strength of this classification system is that it largely relies on the therapy used to treat the complication, which are easily identified in retrospective analyses.
Study size and statistical methods
It was estimated using pilot data that the previous criteria would identify approximately 100 patients. Based on similar studies validating grading systems in other cerebral pathologies, we considered 100 patients sufficient for meaningful analysis [37].
Data were analysed using with SPSS v 20.0 (IBM, Illinois, USA). The mean and standard deviation were calculated for parametric variables, and the median and interquartile ranges were calculated for non-parametric variables. Cohen’s κ was calculated to determine the agreement between the two neurosurgeons grades. The chi-square test was then used to (1) compare the grade against extent of resection and (2) compare the grade against the presence of major complications (Clavian-Dindo greater than 3a). A value of P < 0.05 was considered statistically significant.
Results
Development
Study selection
In all, 99 article titles and abstracts were screened, 25 full articles were considered relevant and further assessed for their eligibility, and 20 articles were ultimately included (Fig. 1).
Study characteristics
The included studies comprised one prospective cohort study, 18 retrospective cohort studies and one case-control study. The patient characteristics, pre-operative MRI features, surgical outcomes and findings are summarised in Table 1.
Grading system
Five features on pre-operative MRI were identified that were found to be predictive of surgical outcome in at least two studies (Table 2): periventricular or deep location, corpus callosum or bilateral location, eloquent location, size and associated oedema. These features were used to develop a grading system for adult patients with supratentorial GBM (Table 3).
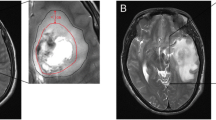
The grading system was designed to be simple, objective and reproducible. Each feature is measured on a standard pre-operative contrast-enhanced T1-weighted MRI: periventricular or deep location if the contrast-enhancing tumour is located within 10 mm of the ventricles; corpus callosum or bilateral location if contrast-enhancing tumour extends into these regions; eloquent if contrast-enhancing tumour extends into motor or sensory cortex, language cortex, insula or basal ganglia; large if the diameter of the contrast-enhancing tumour exceeds 40 mm; and associated oedema if hypo-intensity extends more than 10 mm from contrast-enhancing tumour. All features are weighted equally, with one point assigned if a feature was present and no points if absent. The sum of these features is used to describe lesions as low (0–1 points), moderate (2–3 points) and high complexity (4–5 points). An example of a high-complexity tumour is illustrated in Fig. 2.
The grading system uses a standard pre-operative contrast-enhanced T1-weighted MRI as such imaging is readily available, allowing the grading system to be used in resource-limited settings and in retrospective studies. The radiological definitions of periventricular or deep location, large diameter and associated oedema were drawn from the literature and, where there was discrepancy, using the expert opinion of the senior authors (DN and LT). All features were weighted equally, and the sum of these features is used to describe lesions as low, moderate and high complexities, to ensure that the grading system is as simple as possible and to increase the statistical power for series comparisons.
Validation
Participants and descriptive data
In all, 106 patients were identified with supratentorial GBM who underwent craniotomy and resection. Of these, 18 patients were excluded because their imaging (n = 9) or clinical notes (n = 9) could not be found, and 88 patients were included. The patient demographics are summarised in Table 4.
Outcome data and main results
Pre-operative MRI features are summarised in Table 5. The majority of tumours had a periventricular location (77.3%; 68/88), were greater than 40 mm in diameter (70.5%; 62/88) and had significant associated oedema (69.3%; 61/88). Corpus callosum involvement, or bilateral location, was seen in 33.0% of tumours (29/88), while eloquent location involvement was seen in 43.2% (38/88) of tumours.
Post-operative MRI demonstrated complete resection of contrast-enhancing tumour in 15 patients (17.0%). Three patients (3.4%) had major complications: two had intracerebral haemorrhage, and one had massive pulmonary embolism.
There was a substantial agreement between the two neurosurgeons grading GBM (Cohen’s κ was 0.625; standard error 0.066). The grades and surgical outcomes are summarised in Table 6 and Fig. 3. There was a significant association between grades and extent of resection (p = 0.0007) but not complications (p = 0.4148).
Discussion
Principal findings
Currently, the literature on GBM management contains limited data on relevant surgical anatomy. When such data are available, it is often described in a variable manner, making comparative effectiveness research difficult. This study reports the development and validation of a simple, objective and reproducible grading system for GBM. The proposed grading system allows for the standardised reporting of the five most important features of GBM on pre-operative MRI: periventricular or deep location, corpus callosum or bilateral location, eloquent location, size and associated oedema. Moreover, this grading system was found to be predictive of surgical outcome. High-complexity lesions were significantly less likely to result in complete resection of contrast-enhancing tumour than low-complexity lesions (50.0 versus 3.4%; p = 0.0007). High-complexity lesions were also more likely to result in major complications, though this did not achieve statistical significance (6.9 versus 0%; p = 0.4148).
Comparison with other studies
Each element of the proposed grading system has been previously identified in the literature as affecting surgical outcome. The most consistently reported of these elements is GBM near the ventricles, or deep location, which eight articles found to be associated with reduced extent of resection and overall survival. The resection of deep-seated GBM necessitates pial and subpial transection. Multiple studies have demonstrated that lesions of the deep white matter tracts elicit more severe and permanent neurological deficits than cortical injuries of comparable volume, making the complete resection of contrast-enhancing tumour challenging [18]. Benveniste et al. found that, for tumours under 30 ml in volume, only 77% of those lying adjacent to the ventricles were completely resected, compared to 91% of those lying elsewhere. Emerging technologies such as high-definition fibre tractography and endoscopic port surgery may improve the visualisation and resection of tumours lying near the ventricles [5, 18, 40].
The presence of cerebral oedema associated with GBM is a common feature on pre-operative MRI and is known to affect overall survival. All the patients in our cohort were given high-dose dexamethasone pre-operatively, and in more than two thirds, major oedema persisted on imaging. Major oedema can result in considerable difficulty during surgical resection, obscuring identification of anatomical landmarks and making brain tissue less amenable to manipulation. To this end, evidence suggests that minimal oedema is associated with increased overall survival (albeit only with univariate analysis), while major oedema, extending more than 10 mm from the tumour margin, is associated with reduced survival [21, 27, 35].
It has long been recognised that individual tumour cells frequently spread into the contralateral hemisphere in patients with GBM [14]. However, only a third of patients in our cohort were found to have contrast-enhancing tumour infiltrate the corpus callosum on pre-operative MRI. The surgical resection of lesions within the corpus callosum, particularly the posterior region, can result in major complications such as disconnection syndrome. Infiltration of the corpus callosum is therefore associated with significantly reduced extent of resection, reduced progression-free survival and reduced overall survival [11]. Similarly, tumours extending into both hemispheres are associated with worse overall survival [1, 22].
Larger GBM, assessed by both linear and volumetric parameters, is associated with reduced extent of resection and worse overall survival [4, 8, 10, 16, 33]. A number of metrics have been used to dichotomise tumours according to size, including a tumour volume greater than 30 ml or a maximal diameter greater than 2 or 40 mm [4, 7, 10]. As volumetric and linear metrics have been shown to be comparable and linear metrics are more straightforward to measure on standard MRI, we used a maximal diameter greater than 40 mm in the proposed grading system.
Several studies have identified GBM located within eloquent regions as a negative predictor of extent of resection and overall survival [16, 27, 28, 30, 33, 39]. The regions considered eloquent vary in the literature, but those most commonly included were used in the proposed grading system (motor or sensory cortex, language cortex, insula or basal ganglia). Historically, complete resection of lesions residing within these regions was believed to invariably result in permanent neurological deficits. Recent evidence, however, points to considerable plasticity within these regions, and techniques such as awake craniotomy may yet allow for complete resection of contrast-enhancing tumours in selected cases [40].
Taken together, the five aforementioned pre-operative MRI features have a major impact on surgical outcome. Studies comparing different operative techniques must therefore carefully account for these confounders. Although the overall rate of complete resection in our preliminary validation study was modest compared to the literature (17.0%; 15/88), the rate of complete resection of contrast-enhancing tumour varied widely from 3.4% in high-complexity lesions (4–5 features) to 50.0% in low-complexity lesions (0–1 features). In the largest prospective study on patients with GBM, only 36% of patients were found to have their complete resection of contrast-enhancing tumour using conventional microsurgical techniques [38]. Other studies, which report a rate of complete resection of contrast-enhancing tumour in up to 90% of cases, are usually retrospective, often exclude patients in whom complete resection is deemed impossible such as those involving the corpus callosum or eloquent brain, and may combine near total and gross total resection in their analyses [36].
To the best of our knowledge, the proposed grading system is the first to use features on pre-operative MRI to predict the surgical outcome of adult patients undergoing craniotomy for GBM. However, several other systems have been described that use a combination of demographic, clinical and radiological features to predict overall survival. Chaichana et al. developed a system that included the patient’s Karnofsky Performance Status, age, the presence of a motor deficit, the presence of a language deficit and periventricular tumour location. This system was initially validated on 393 prospective cases at one centre and then later on 334 patients across three centres [10]. Park et al. developed a similar system using the Karnofsky Performance Status, tumour volume and tumour location within eloquent or critical regions, which was validated on a retrospective cohort of 34 consecutive patients at one centre [33]. Although these systems are useful, the proposed grading system differs in focusing on anatomical features of GBM that influence the operative complexity and therefore the extent of resection and likelihood of major complications.
Limitations
There were several limitations to the validation of the proposed grading system. First, the retrospective nature of the study introduces the possibility of, for example, selection bias. The accurate and precise recording of patient complications, in particular, is difficult in such study designs. Second, the single-centre design makes it difficult to generalise the findings to different centres with different patient populations, case selection and operative techniques. Third, the study was powered to validate the grading system with respect to extent of resection, but not major complications. Finally, the study did not measure important other surgical outcomes such as progression-free survival and overall survival. These limitations might be addressed through prospective, multicentre and larger studies.
Conclusions
The proposed grading system may allow for the standardised communication of anatomical features of GBM identified on pre-operative MRI. While the grading system may not capture all the elements that contribute to the complexity of individual cases, we believe that it captures the most relevant characteristics in a reproducible manner. We hope that use of this grading system in clinical practice and in the literature will enable more standardisation of clinical care and more meaningful comparisons of clinical studies.
Author contributions
HJM and AHH were involved in the study conception, acquisition of data, analysis of data and drafting of the manuscript. SW and SJC were involved in the acquisition of data, analysis of data and drafting of the manuscript. DN and LT were involved in the study conception and critical revision of the manuscript.
References
Adeberg S, Bostel T, Konig L, Welzel T, Debus J, Combs SE (2014) A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol 9:95. doi:10.1186/1748-717X-9-95
Balog J, Sasi-Szabo L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, Mirnezami R, Dezso B, Damjanovich L, Darzi A et al (2013) Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med 5. doi:10.1126/scitranslmed.3005623
Barone DG, Lawrie TA, Hart MG (2014) Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev 1:CD009685. doi:10.1002/14651858.CD009685.pub2
Benveniste R, Germano IM (2003) Evaluation of factors predicting accurate resection of high-grade gliomas by using frameless image-guided stereotactic guidance. Neurosurg Focus 14(2):e5
Bodily L, Mintz AH, Engh J (2013) Combined awake craniotomy with endoscopic port surgery for resection of a deep-seated temporal lobe glioma: a case report. Case Rep Med 2013:401359. doi:10.1155/2013/401359
Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A (2008) Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol 89(2):219–224
Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A (2010) A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg 112(5):997–1004. doi:10.3171/2009.9.JNS09805
Chaichana KL, Chaichana KK, Olivi A, Weingart JD, Bennett R, Brem H, Quinones-Hinojosa A (2011) Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg 114(3):587–594. doi:10.3171/2010.8.JNS1081
Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, Li G, Harsh GR IV, Thompson RC, Lim M, Quinones-Hinojosa (2013a) A Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci 20(10):1422–1426
Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R, Weingart JD, Olivi A, Gallia GL, Lim M, Brem H, Quinones-Hinojosa A (2013b) Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci 20(6):818–823. doi:10.1016/j.jocn.2012.07.016
Chen KT, Wu TW, Chuang CC, Hsu YH, Hsu PW, Huang YC, Lin TK, Chang CN, Lee ST, Wu CT et al (2015) Corpus callosum involvement and postoperative outcomes of patients with gliomas. J Neuro-Oncol 124(2):207–214. doi:10.1007/s11060-015-1823-0
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C et al (2009) The Clavien-Dindo classification of surgical complications five-year experience. Ann Surg 250:187–196. doi:10.1097/SLA.0b013e3181b13ca2
Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A, Grp I (2013) IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. Bmj-British Medical Journal 346. doi:10.1136/Bmj.F2820
Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA 90:823–825
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications—a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. doi:10.1097/01.sla.0000133083.54934.ae
Durmaz R, Erken S, Arslantas A, Atasoy MA, Bal C, Tel E (1997) Management of glioblastoma multiforme: with special reference to recurrence. Clin Neurol Neurosurg 99(2):117–123
Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG, Grp I (2013) IDEAL framework for surgical innovation 2: observational studies in the exploration assessment stages. Bmj-British Medical Journal 346. doi:10.1136/Bmj.F3011
Fernandez-Miranda JC, Engh JA, Pathak SK, Madhok R, Boada FE, Schneider W, Kassam AB (2010) High-definition fiber tracking guidance for intraparenchymal endoscopic port surgery. J Neurosurg 113:990–999. doi:10.3171/2009.10.JNS09933
Foersch S, Heimann A, Ayyad A, Spoden GA, Florin L, Mpoukouvalas K, Kiesslich R, Kempski O, Goetz M, Charalampaki P (2012) Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. PLoS One 7:e41760. doi:10.1371/journal.pone.0041760
Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F, Lango T, Unsgard G (2000) SonoWand, an ultrasound-based neuronavigation system. Neurosurgery 47:1373–1379 discussion 1379-1380
Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27(1):65–73
Helseth R, Helseth E, Johannesen TB, Langberg CW, Lote K, Ronning P, Scheie D, Vik A, Meling TR (2010) Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand 122(3):159–167. doi:10.1111/j.1600-0404.2010.01350.x
Jain R, Poisson LM, Gutman D, Scarpace L, Hwang SN, Holder CA, Wintermark M, Rao A, Colen RR, Kirby J, Freymann J, Jaffe CC, Mikkelsen T, Flanders A (2014) Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: focus on the nonenhancing component of the tumor. Radiology 272(2):484–493
Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K, Bernstein L, Guiot MC, Petrecca K, Leblond F (2015) Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med 7. doi:10.1126/scitranslmed.aaa2384
Konglund A, Helseth R, Lund-Johansen M, Helseth E, Meling TR (2013) Surgery for high-grade gliomas in the aging. Acta Neurol Scand 128(3):185–193
Kubben PL, ter Meulen KJ, Schijns OEMG, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12:1062–1070. doi:10.1016/S1470-2045(11)70130-9
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198. doi:10.3171/jns.2001.95.2.0190
Li SW, Qiu XG, Chen BS, Zhang W, Ren H, Wang ZC, Jiang T (2009) Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin Med J (Engl) 122(11):1245–1249
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124:977–988. doi:10.3171/2015.5.JNS142087
Ma X, Lv Y, Liu J, Wang D, Huang Q, Wang X, Li G, Xu S, Li X (2009) Survival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in China. J Clin Neurosci 16(12):1595–1598. doi:10.1016/j.jocn.2009.02.036
McCulloch P, Cook JA, Altman DG, Heneghan C, Diener MK, Grp I (2013) IDEAL framework for surgical innovation 1: the idea and development stages. Bmj-British Medical Journal 346. doi:10.1136/Bmj.F3012
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 339:b2535. doi:10.1136/bmj.b2535
Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, Olsen Bailey N, Kreisl TN, Iwamoto FM, Sul J et al (2010) Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol 28(24):3838–3843. doi:10.1200/JCO.2010.30.0582
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. doi:10.3171/2011.2.JNS1099810.3171/2011.7.JNS10238
Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D, Heinzl H, Lahrmann H, Marosi C, Grisold W (2009) Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol 16(7):874–878. doi:10.1111/j.1468-1331.2009.02613.x
Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, Stieglitz L, Raabe A (2012) Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery 71:927–935 . doi:10.1227/NEU.0b013e31826d1e6bdiscussion 935-926
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. doi:10.1016/S1470-2045(06)70665-9
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–576 . doi:10.1227/01.neu.0000317304.31579.17 discussion 564–576
Taylor MD, Bernstein M (1999) Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg 90:35–41. doi:10.3171/jns.1999.90.1.0035
Tejada-Solis S, Aldave-Orzaiz G, Pay-Valverde E, Marigil-Sanchez M, Idoate-Gastearena MA, Diez-Valle R (2012) Prognostic value of ventricular wall fluorescence during 5-aminolevulinic-guided surgery for glioblastoma. Acta Neurochir Wien 154(11):1997–2002
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. doi:10.1016/S0140-6736(07)61602-X
Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J (2003) Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir 145:5–10. doi:10.1007/s00701-002-1030-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
H.J. Marcus was supported by an Imperial College Wellcome Trust Clinical Fellowship.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethical approval was obtained from the Joint Research Compliance Office (JRCO), Imperial College London.
Informed consent
Informed consent was not sought as a retrospective study design was used.
Copyright
The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors a worldwide licence to the publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display and store the contribution; (ii) translate the contribution into other languages, create adaptations and reprints, include within collections and create summaries, extracts and/or abstracts of the contribution; (iii) create any other derivative work(s) based on the contribution; (iv) exploit all subsidiary rights in the contribution and (v) the inclusion of electronic links from the contribution to third-party material wherever it may be located; and (vi) licence any third party to do any or all of the above.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marcus, H.J., Williams, S., Hughes-Hallett, A. et al. Predicting surgical outcome in patients with glioblastoma multiforme using pre-operative magnetic resonance imaging: development and preliminary validation of a grading system. Neurosurg Rev 40, 621–631 (2017). https://doi.org/10.1007/s10143-017-0817-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-017-0817-0