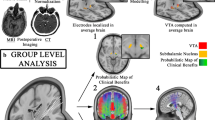
Abstract
Recent advances in imaging permit radiologic identification of target structures for deep brain stimulation (DBS) for movement disorders. However, these methods cannot detect the internal subdivision and thus cannot determine the appropriate DBS target located within those subdivisions. The aim of this study is to provide a straightforward method to obtain an optimized target (OT) within DBS target nuclei using a widely available navigation system. We used T1- and T2-weighted images, fluid-attenuated inversion recovery (FLAIR) sequence, and diffusion tensor imaging (DTI) of nine patients operated for DBS in our center. Using the StealthViz® software, we segmented the targeted deep structures (subcortical targets) and the anatomically identifiable areas to which these target nuclei were connected (projection areas). We generated fiber tracts from the projection areas. By identifying their intersections with the subcortical targets, we obtained an OT within the DBS target nuclei. We computed the distances from the clinically effective electrode contacts (CEEC) to the OT obtained by our method and the targets provided by the atlas. These distances were compared using a Wilcoxon signed-rank test, with p < 0.05 considered statistically significant. We were able to identify OT coincident with the motor part of the subthalamic nucleus and the ventral intermediate nucleus. We clinically tested the results and found that the CEEC were significantly more closely related to the OT than with the targets obtained by the atlas. Our present results show that this novel method permits optimization of the stimulation site within the internal subdivisions of target nuclei for DBS.








Similar content being viewed by others
References
Abosch A, Timmermann L, Bartley S, Rietkerk HG, Whiting D, Connolly PJ, Lanctin D, Hariz MI (2013) An international survey of deep brain stimulation procedural steps. Stereotact Funct Neurosurg 91(1):1–11
Abosch A, Yacoub E, Ugurbil K, Harel N (2010) An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery 67(6):1745–1756, discussion 1756
Akakin A, Peris-Celda M, Kilic T, Seker A, Gutierrez-Martin A, Rhoton A (2014) The dentate nucleus and its projection system in the human cerebellum: the dentate nucleus microsurgical anatomical study. Neurosurgery 74(4):401–425
Anderson JS, Dhatt HS, Ferguson MA, Lopez-Larson M, Schrock LE, House PA, Yurgelun-Todd D (2011) Functional connectivity targeting for deep brain stimulation in essential tremor. AJNR Am J Neuroradiol 32(10):1963–1968
Andrade-Souza YM, Schwalb JM, Hamani C, Eltahawy H, Hoque T, Saint-Cyr J, Lozano AM (2005) Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson’s disease. Neurosurgery 56(2 Suppl):360–368, discussion 360–8
Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H (2007) Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage 37(3):694–705
Barbe MT, Liebhart L, Runge M, Deyng J, Florin E, Wojtecki L, Schnitzler A, Allert N, Sturm V, Fink GR, Maarouf M, Timmermann L (2011) Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: stimulation below intercommissural line is more efficient but equally effective as stimulation above. Exp Neurol 230(1):131–137
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15(7–8):456–467
Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R (2011) Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage 55(4):1728–1738
Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34(1):144–155
Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6(7):750–757
Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50(5):1077–1088
Benabid AL, Torres N (2012) New targets for DBS. Parkinsonism Relat Disord 18(Suppl 1):S21–S23
Benarroch EE (2008) Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology 70(21):1991–1995
Blomstedt P, Sandvik U, Tisch S (2010) Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord 25(10):1350–1356
Brunenberg EJL, Moeskops P, Backes WH, Pollo C, Cammoun L, Vilanova A, Janssen MLF, Visser-Vandewalle VERM, ter Haar Romeny BM, Thiran J-P, Platel B (2012) Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PLoS One 7(6), e39061
Chabardès S, Polosan M, Krack P, Bastin J, Krainik A, David O, Bougerol T, Benabid AL (2012) Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg 80:1–8
Chowdhury R, Lambert C, Dolan RJ, Düzel E (2013) Parcellation of the human substantia nigra based on anatomical connectivity to the striatum. Neuroimage 81:191–198
Coenen VA, Allert N, Mädler B (2011) A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochir (Wien) 153(8):1579–1585, discussion 1585
Coenen VA, Mädler B, Schiffbauer H, Urbach H, Allert N (2011) Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery 68(4):1069–1075, discussion 1075–6
Coenen VA, Prescher A, Schmidt T, Picozzi P, Gielen FLH (2008) What is dorso-lateral in the subthalamic nucleus (STN)?—a topographic and anatomical consideration on the ambiguous description of today’s primary target for deep brain stimulation (DBS) surgery. Acta Neurochir (Wien) 150(11):1163–1165, discussion 1165
Coenen VA, Schlaepfer TE, Allert N, Mädler B (2012) Diffusion tensor imaging and neuromodulation: DTI as key technology for deep brain stimulation. Int Rev Neurobiol 107:207–234
DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64(1):20–24
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980
Destrieux C, Fischl B, Dale A, Halgren E (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53(1):1–15
Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ (2008) Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28(28):7143–7152
Elias WJ, Zheng ZA, Domer P, Quigg M, Pouratian N (2012) Validation of connectivity-based thalamic segmentation with direct electrophysiologic recordings from human sensory thalamus. Neuroimage 59(3):2025–2034
Farquharson S, Tournier J-D, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A (2013) White matter fiber tractography: why we need to move beyond DTI. J Neurosurg 118(6):1367–1377
Fernandez-Miranda JC, Pathak S, Engh J, Jarbo K, Verstynen T, Yeh F-C, Wang Y, Mintz A, Boada F, Schneider W, Friedlander R (2012) High-definition fiber tractography of the human brain: neuroanatomical validation and neurosurgical applications. Neurosurgery 71(2):430–453
Fuster JM (2008) The prefrontal cortex, Fourth ed. 7–44
Guridi J, Rodríguez-Oroz MC, Ramos E, Linazasoro G, Obeso JA (2002) Discrepancy between imaging and neurophysiology in deep brain stimulation of medial pallidum and subthalamic nucleus in Parkinson’s disease. Neurologia 17(4):183–192
Gutman DA, Holtzheimer PE, Behrens TEJ, Johansen-Berg H, Mayberg HS (2009) A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65(4):276–282
Hamani C, Dostrovsky JO, Lozano AM (2006) The motor thalamus in neurosurgery. Neurosurgery 58(1):146–158
Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM (2004) The subthalamic nucleus in the context of movement disorders. Brain 127(Pt 1):4–20
Haynes WI, Mallet L (2010) High-frequency stimulation of deep brain structures in obsessive-compulsive disorder: the search for a valid circuit. Eur J Neurosci 32(7):1118–1127
Hyam JA, Owen SLF, Kringelbach ML, Jenkinson N, Stein JF, Green AL, Aziz TZ (2012) Contrasting connectivity of the ventralis intermedius and ventralis oralis posterior nuclei of the motor thalamus demonstrated by probabilistic tractography. Neurosurgery 70(1):162–169, discussion 169
Jiang H, van Zijl PCM, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81(2):106–116
Klein JC, Rushworth MFS, Behrens TEJ, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H (2010) Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage 51(2):555–564
Kopell BH, Greenberg BD (2008) Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev 32(3):408–422
Kopell BH, Rezai AR, Chang JW, Vitek JL (2006) Anatomy and physiology of the basal ganglia: implications for deep brain stimulation for Parkinson’s disease. Mov Disord 21(Suppl 1):S238–S246
Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, Draganski B, Ashburner J, Frackowiak R (2012) Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage 60(1):83–94
Lehéricy S, Ducros M, Krainik A, Francois C, Van de Moortele P-F, Ugurbil K, Kim D-S (2004) 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex 14(12):1302–1309
Lenglet C, Abosch A, Yacoub E, De Martino F, Sapiro G, Harel N (2012) Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7 T MRI. PLoS One 7(1), e29153
Maruyama K, Koga T, Kamada K, Ota T, Itoh D, Ino K, Igaki H, Aoki S, Masutani Y, Shin M, Saito N (2009) Arcuate fasciculus tractography integrated into Gamma Knife surgery. J Neurosurg 111(3):520–526
Mori S, van Zijl PCM (2002) Fiber tracking: principles and strategies—a technical review. NMR Biomed 15(7–8):468–480
Nieuwenhuys R, Voogd J, Van Huijzen C (2008) The human central nervous system. 253–286, 427–653
Okun MS, Gallo BV, Mandybur G et al (2012) Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 11(2):140–149
Ongür D, Ferry AT, Price JL (2003) Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460(3):425–449
Papavassiliou E, Rau G, Heath S, Abosch A, Barbaro NM, Larson PS, Lamborn K, Starr PA (2004) Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery 54(5):1120–1130
Parent A, Lévesque M, Parent M (2001) A re-evaluation of the current model of the basal ganglia. Parkinsonism Relat Disord 7(3):193–198
Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA (2011) Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. J Neurosurg 115(5):995–1004
Richter EO, Hoque T, Halliday W, Lozano AM, Saint-Cyr JA (2004) Determining the position and size of the subthalamic nucleus based on magnetic resonance imaging results in patients with advanced Parkinson disease. J Neurosurg 100(3):541–546
Rodriguez-Oroz MC, López-Azcárate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, Guridi J, Artieda J, Obeso JA (2011) Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 134(Pt 1):36–49
Schaltenbrand G, Wahren W (1977) Atlas for stereotaxy of the human brain. Second ed. Thieme, Stuttgart
Sedrak M, Gorgulho A, Bari A, Behnke E, Frew A, Gevorkyan I, Pouratian N, DeSalles A (2010) Diffusion tensor imaging (DTI) and colored fractional anisotropy (FA) mapping of the subthalamic nucleus (STN) and the globus pallidus interna (GPi). Acta Neurochir (Wien) 152(12):2079–2084
Sedrak M, Gorgulho A, Frew A, Behnke E, DeSalles A, Pouratian N (2011) Diffusion tensor imaging and colored fractional anisotropy mapping of the ventralis intermedius nucleus of the thalamus. Neurosurgery 69(5):1124–1129, discussion 1129–30
Shenai MB, Romeo A, Walker HC, Guthrie S, Watts RL, Guthrie BL (2015) Spatial topographies of unilateral subthalamic nucleus deep brain stimulation efficacy for ipsilateral, contralateral, midline, and total Parkinson disease motor symptoms. Neurosurgery 00(00):8–14
Stancanello J, Muacevic A, Sebastiano F, Modugno N, Cerveri P, Ferrigno G, Uggeri F, Romanelli P (2008) 3 T MRI evaluation of the accuracy of atlas-based subthalamic nucleus identification. Med Phys 35(7):3069–3077
Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J (2003) The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J Chem Neuroanat 26(4):293–299
Sudhyadhom A, McGregor K, Okun MS, Foote KD, Trinastic J, Crosson B, Bova FJ (2013) Delineation of motor and somatosensory thalamic subregions utilizing probabilistic diffusion tractography and electrophysiology. J Magn Reson Imaging 37(3):600–609
Tamura M, Hayashi M, Konishi Y, Tamura N, Regis J, Mangin JF, Taira T, Okada Y, Muragaki Y, Iseki H (2013) Advanced image coregistration within the Leksell workstation for the planning of glioma surgery: initial experience. J Neurol Surg Rep 74(2):118–122
Toda H, Sawamoto N, Hanakawa T, Saiki H, Matsumoto S, Okumura R, Ishikawa M, Fukuyama H, Hashimoto N (2009) A novel composite targeting method using high-field magnetic resonance imaging for subthalamic nucleus deep brain stimulation. J Neurosurg 111(4):737–745
Tomassini V, Jbabdi S, Klein JC, Behrens TEJ, Pozzilli C, Matthews PM, Rushworth MFS, Johansen-Berg H (2007) Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci 27(38):10259–10269
Utter AA, Basso MA (2008) The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev 32(3):333–342
Vaillancourt DE, Sturman MM, Verhagen Metman L, Bakay RA, Corcos DM (2003) Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology 61(7):919–925
Vertinsky AT, Coenen VA, Lang DJ, Kolind S, Honey CR, Li D, Rauscher A (2009) Localization of the subthalamic nucleus: optimization with susceptibility-weighted phase MR imaging. AJNR Am J Neuroradiol 30(9):1717–1724
Wodarg F, Herzog J, Reese R, Falk D, Pinsker MO, Steigerwald F, Jansen O, Deuschl G, Mehdorn HM, Volkmann J (2012) Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord 27(7):874–879
Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T (2009) MR tractography: a review of its clinical applications. Magn Reson Med Sci 8(4):165–174
Yang Y, Raine A (2009) Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res 174(2):81–88
Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tandé D, Karachi C, Ayache N, Cornu P, Agid Y (2007) A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. Neuroimage 34(2):618–638
Zrinzo L (2010) The role of imaging in the surgical treatment of movement disorders. Neuroimaging Clin N Am 20(1):125–140
Acknowledgments
This work was supported by Instituto de Salud Carlos III, grant number PI10/1932. The authors thank Dr. Juan Alvarez-Linera for the image acquisition, Juan Sobrado and Julio Gonzalez for the technical support in the software, and Mrs. Ingrid Carranza for the support and assistance in the figures, tables, and illustrations.
Disclosures
The authors have no personal financial or institutional interest in any of the materials or devices described in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Tetsuya Goto, Matsumoto, Japan
The authors reported the useful target measuring method in deep brain stimulation surgery guided by MRI tractography. Their methodology looks reasonable and agreeable. They concluded that their advocating target was superior to using atlas-based target (x −12 mm, y −3 mm, z −4 mm from AC-PC line) because it was nearer to the most clinically effective electrode contact point than atlas-based target.
When the stereotactic surgery guided by microrecording is performed, the initial target should be determined by not only the final target point in the dorsolateral STN but also the insertion point, trajectory angle, ventricle size, and thickness of thalamus. The most clinically effective electrode contact point is not also the initial target because it is decided after checking the effectiveness of treatment and the complications of side effects. The authors moved the target two or three times, although they were guided by their method. The selection of the initial target might be discussed by the number of times of the trajectory.
If the direct visualization of the STN is possible by using their method, it may be superior to conventional technique. These techniques will shift the paradigm from the microrecording-guided to radiographic-guided stereotactic surgery. They will reduce the number of times of the trajectory, risk of hemorrhage, and operation time.
Peter Grunert, Homburg/Saar, Germany
An intrinsic problem in functional stereotaxy is the fact that in most of the cases, the target is not directly visible in the images neither in ventriculography nor in CT or in MRI. Therefore, indirect methods based on a human atlas have been developed to determine the target point in relation to defined anatomical landmarks such as the anterior and posterior commissure. Additionally, intraoperative microelectrode recording and electric stimulation were routinely intraoperatively applied during deep brain stimulation to optimize the target for the final placement of the electrode. The authors in this contribution proposed a new method for optimizing the target point by visualization of the afferent or efferent tracts to or from the target area. For the STN, they visualized the connections of this nucleus to the cortical motor and several premotor areas. For the VIM nucleus in the thalamus, they were able to demonstrate the efferent fibers from dentate and ruber nucleus to the area of the VIM. This was achieved in MRI images by the meanwhile established method of fiber tracking. Despite several technical limitations of this method, the authors could show that their optimized target calculation based on tractography was statically more close to the final target established by electrophysiological methods than the calculation based on a stereotactic atlas.
I think this is a very interesting and original contribution with great potential in the future. The tractography with the visualization of the well-known anatomical afferent and efferent connections seems to be a very logical and promising method to optimize the target even within the target area. In the future, with better image resolution, tractography may make the time-consuming electrophysiological testing superfluous. However, at this point of development, in particular for small target areas, the electrophysiological investigations are still indispensible.
Jürgen Voges, Magdeburg, Germany
This manuscript describes a procedure using deterministic tractography (DTI) to improve stereotactic targeting. The integration of DTI into stereotactic treatment planning protocols for DBS surgery seems logical because it is widely accepted that large fibers originating in or projecting onto the stimulated area play a prominent role in mediating the beneficial effects of neurostimulation. Referred to the here examined targets, crucial fiber tracts are the “hyperdirect pathway” in the case of the subthalamic nucleus or the “dentatorubrothalamic tract” (DRT) when the ventral intermediate thalamic nucleus is electrically stimulated. If direct targeting of fiber tracts instead of relais nuclei will improve the clinical outcome is not yet clearly defined. Schlaier and collaborators, for instance, addressing intraoperative tremor improvement as a function of the spatial relationship of active electrode contacts and the DRT, reported that the distance to this fiber tract had no impact on the outcome (1). The findings of Coenen et al., in contrast, displayed a trend for better tremor response when active electrode contacts projected onto the DRT in comparison to those contacts located at the anterior border of this tract, but this difference was statistically not significant (2).
General concerns, when using clinical tractography, refer to the anatomic accuracy of this method. Thomas and colleagues investigated in-depth the assumption that the combination of high-resolution diffusion-weighted imaging and sophisticated diffusion modeling approaches may provide anatomically correct connectivity maps of the brain. Comparing the “visualized” connections with those derived from tracer studies—the “gold standard”—this group demonstrated that suboptimal information accuracy results from inherent methodological limitations of tractography. According to their conclusions, comprehensive methodological modifications are required to overcome these limitations (3). Related to stereotactic treatment planning, tractography cannot replace electrophysiology and/or intraoperative clinical testing at that time, and keeping the aforementioned methodological problems in reference, it is recommended to use DTI skeptically.
References
1. Schlaier J, Anthofer J, Steib K, Fellner C, Rothenfusser E, Brawanski A, Lange M. Deep brain stimulation for essential tremor: targeting the dentato-rubro-thalamic tract? Neuromodulation 2014.
2. Coenen VA, Allert N, Paus S, Kronenburger M, Urbach H, Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 2014;75:657–670.
3. Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A 2014;111:16574–16579.
Rights and permissions
About this article
Cite this article
Avecillas-Chasin, J.M., Alonso-Frech, F., Parras, O. et al. Assessment of a method to determine deep brain stimulation targets using deterministic tractography in a navigation system. Neurosurg Rev 38, 739–751 (2015). https://doi.org/10.1007/s10143-015-0643-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0643-1