Abstract
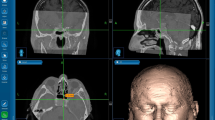
The utility of a neuronavigation system in the transsphenoidal surgery of pituitary macroadenoma was evaluated, and improvement of the surgical outcome is discussed. From 1997 to 2003, a total of 63 patients (male:female=41:22, mean age 58.3 years) with pituitary macroadenoma were treated surgically via transsphenoidal approach using a neuronavigation system in Beijing Tiantan Hospital. Image data for computed tomography or magnetic resonance were obtained and analyzed by the navigation system to make a three-dmensional reconstruction. During the operation, the tumor and its surrounding structures of the sellar region could be located accurately at any time. Among these cases, the tumors were removed totally in 26 cases (41.3%), subtotally in 36 cases (57.1%) and partially in one case (1.6%). Postoperative neurological complications occurred in 15 cases (23.8%). One patient died, the operative mortality was 1.6%. During the operation, the accuracy of the neuronavigation system was 2.3±1.1 mm. The neuronavigation system is quite helpful for transsphenoidal surgery of pituitary macroadenoma. Its accuracy of location is very useful and important in determining anatomical structure and protecting normal tissues and vessels. Moreover, fluoroscopy was not required during the surgery.

Similar content being viewed by others
References
Bohinski RJ, Warnick RE, Gaskill-Shipley MF et al (2001) Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery 49:1133–1144
Du GH, Mao Y, Zhou LF (2001) Use of neuronavigation system inmicrosurgery of pituitary adenoma. Chi J Minim Invasive Neurosurg 6:65–68
Elias WJ, Chadduck JB, Alden TD et al (1999) Frameless stereotaxy for transsphenoidal surgery. Neurosurgery 45:271–275
He DS, Chen MZ, Wang HJ (2002) Application of neuronavigation system in transsphenoidal surgery. Stereotaxy Funct Neurosurg 15:32–33
Jane JA, Thapar K, Kaptain GJ et al (2002) Pituitary surgery: transsphenoidal approach. Neurosurgery 51:435–444
Kawamata T, Iseki H, Shibasaki T et al (2002) Endoscopic augmented reality navigation system for Endonasal transsphenoidal surgery to treat Pituitary Tumors: technical note. Neurosurgery 50:1393–1397
Kajiwara K, Nishizaki T, Ohmoto Y, Nomura S, Suzuki M (2003) Image-guided transsphenoidal surgery for pituitary lesions using Mehrkoordinaten Manipulator (MKM) navigation system. Minim Invasive Neurosurg 46:78–81
Knosp E, Steiner E, Kitz K et al (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33:610–618
Lasio G, Ferroli P, Felisati G et al (2002) Image-guided endoscopic transnasal removal of recurrent pituitary adenomas. Neurosurgery 51:132–137
Li G, Jia DZ, Li XG (2003) Transnasal transsphenoidal pituitary surgery. Acta Shandong Univ (med edn) 41:508–510
Nimsky Ch, Rachinger J, Iro H, Fahlbusch R (2004) Adaptation of a hexapod-based robotic system for extended endoscope-assisted transsphenoidal skull base surgery. Minim Invasive Neurosurg 47:41–46
Song DL, Du GH, Bao WM (2001) Neuronavigation assisted pituitary adenoma operations. Chi J Clin Neurosurg 6:204–206
Wang R, Zhao JZ, Wang DJ et al (2002) Imaging shifting in navagation operations. Beijing Med 24:155–157
Yang H, Liu SY, Liu HP et al (2003) Neuroendoscopy and image-guided transsphenoidal pituitary surgery. Acta Third Military Medical Univ 25:1792–1794
Zhao JZ, Cao Y, Lu Z et al (2001) Frameless stereotaxy operations in minimally invasive neurosurgery. Chi Med J 81:1042–1045
Zhao YL, Wang CC, Zhao JZ et al (1998) Clinical application of neuronagivation system in neurosurgical operations: 55 cases reports. Chi J Neurosurg 14:198–202
Zou YJ, Liu HY, Chang Y (2000) Application of navigation system in transnasal transsphenoidal pituitary surgery. Stereotaxy Funct Neurosurg 13:220–221
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Dattatraya Muzumdar
Zhao et al. describe their experience in the clinical application of a neuronavigation system in transphenoidal surgery for pituitary adenoma. They conclude that it is a useful tool in determining the location of anatomical structures and protecting normal tissues and blood vessels. Further, they mention that intraoperative fluouroscopy will not be required during the surgery.
Intraoperative fluoroscopy is still being used in localization of the sphenoid sinus and sella during transsphenoidal surgery and has proved to be extremely useful. However, with the advent of modern technology, the neuronavigation system has been introduced in practice to achieve a similar desirable result. In addition, the relationship of instruments to anatomic structures in a mediolateral direction can be obtained. It may also be an important advance in improving patient safety and in reducing cumulative and potentially dangerous X-ray exposure to the surgeon. It could prove particularly valuable in resurgery for recurrent or residual pituitary tumors, severe demineralization of the cranial base, poor pneumatization of the sphenoid, or bony congenital parasellar anomalies and visualization of cavernous sinus structures. The use of intraoperative fluoroscopy is minimal in experienced hands as they are rather guided by the vomer to confirm the midline and the surgery is performed in less time. In general, with the relatively easy availability of neuronavigation systems, their clinical application in transphenoidal surgery would be routine in the future and it is now mandatory for the pituitary surgeon to familarise and achieve the required technical expertise in operating a neuronavigation system. Another advantage would be that the operation would be safer and help boost the confidence level of the aspiring pituitary surgeon to conduct a smooth operation. The major drawback of the neuronavigation system is the image shifts occurring in the residual tumor as resection proceeds and following descent of the dipahragma sellae. However, it can be obviated by coupling the navigation system with intraoperative MR imaging, computed tomography and endoscopy to gauge the completeness of tumor excision. In developing countries, there would be an added need to justify the cost benefit evaluation of these new developments.
Christopher Nimsky, Michael Buchfelder, Erlangen, Germany
Yanli Zhao et al. report on a series of 63 patients that were operated via the transsphenoidal route in which they had applied pointer-based navigation.
Several anatomical landmarks facilitate orientation in standard transsphenoidal surgery. Nevertheless, the introduction of X-ray fluoroscopy by Hardy and Wigser in 1965 further facilitated orientation in the surgical field and enhanced surgical safety. However, only bony structures of the skull are visible with the use of an image intensifier. At least it allows an anatomical orientation of the surgeon by visualization of radioopaque instruments such as steel drills, curettes and forceps in relation to the bony skull base. It is generally also used to direct the nasal speculum towards the sella, thus minimizing the effort of dissection. The major disadvantage is that it does not directly depict soft tissues. However, it is still the most frequently practiced method for immediate orientation in the surgical field in transsphenoidal surgery, especially compared with the application of navigation systems or CT- or MR-based intraoperative imaging, since the necessary equipment is anyway required for a variety of other standard neurosurgical operations and thus is virtually available in every neurosurgical department.
Minimizing tissue trauma is one of the major advances of minimal invasive surgery. However, in many procedures only large exposures allow the identification of a variety of structures, which aid in defining the localisation of the operative field. Thus, with less invasive surgical procedure, less anatomical landmarks are available which would help for proper orientation. Consequently, a need is created for having additional data available that would help to let the surgeon know where exactly his instruments are placed in relation to the individual anatomical situation of the patient.
The development of technologies for image-guided surgery was one of the most major impact innovations of the past decade. One of the major advantages of these navigation systems is that selected anatomical landmarks, vascular structures, tumour dimensions and other targets can be segmented and finally superimposed with the surgeon’s field of view. Thus, potential operative hazards can be readily recognized by the surgeon while he is carrying on with the dissection. There is no clear-cut distinction between intraoperative imaging and navigation, since even the least sophisticated intraoperative imaging usually allows some navigation. Certainly, such systems are not absolutely necessary nor even desirable for routine pituitary surgery. On the other hand, they can be extremely helpful in selected instances. Unusual anatomical variations, prior pituitary surgery, and specifically large tumours with unusual extensions are considered indicative for image-guided surgery.
Vascular anomalies, such as kinks and coils of the carotid artery into the sella, are the nightmare of the transsphenoidal surgeon and thus represent situations in which even the most experienced surgeon appreciates the advantage of image guidance. Ideally the segmented vascular structures are projected into the operative situs from the very beginning of the procedure. Another potential application is a poorly pneumatized sphenoid sinus. Navigation may be used for drilling through the osseous portions of the skull base and a safer procedure can result from the use of this technology, with a better exposure of the tumour and gland.
There are pointer-related systems available, which are mainly devised for localisation of a given structure that is encountered during dissection, in the radiological image. These also allow the definition of the ideal trajectory to a given target. We believe, that microscope-based navigation systems, which allow the direct visualisation of segmented structures are even more to the comfort of the surgeon. It is obvious, that surgeons less familiar with the procedure will appreciate the use of the systems even during standard procedures. Furthermore, navigation combined with endoscopic surgery adds precisional localisation to a minimally invasive procedure. The more minimal the exposure, the greater is the need for technology that supports orientation. However, in addition to the relatively high costs of the machinery, the data acquisition for a navigation procedure is relatively expensive and time-consuming, since it requires image data acquisition with fiducials, i.e. the performance of another MR and CT relatively close prior to surgery and referencing of the systems with the reality of the patients head just before the operation itself is being commenced. These manoeuvres frequently exceed the duration of the actual surgery itself, particularly when the latter is carried out by experienced staff.
Ulrich Sure, Marburg
Zhao et al. report on their extensive experience with pituitary adenoma. Their group treated 2,249 patients in only seven years. In their paper, they focus on their experience with 63 macroadenomas that were operated upon under the aid of varying neuronavigational devices.
The authors describe their technique of the surgical intervention in detail. Their neurosurgical procedure is similar to that which is used in most internationally recognized centres that treat pituitary adenomas. However, in my opinion the substitution of bone for the nasal septum and sponge for the sellar floor in case of a CSF leak, as described by the authors, should be usually performed by a faszia lata patch and/or antilogous fat tissue.
The authors mention the better clinical results for navigated patients when compared with those who were not navigated (frequency of CSF-leak), but fail to present their exact data on that highly interesting issue. Furthermore, they mention that they used at least five bony landmarks in a subset of patients and evaluated the real registration error of the systems. It would be highly interesting to know these landmarks, and the technique for how they could evaluate the error in sub-millimetre precision if the thickness of their scans was usually 1.5 mm, as they reported.
In their discussion, they mention that the diaphragm might shift during surgery, which is in fact a matter of the so-called brainshift (which is more a shift of the cisterns in this case). They correctly stress that the surgeons should be aware of this fact, particularly when treating large suprasellar adenomas via a transsphenoidal approach. I do not know whether neuronavigation really decreases the number of cases that are necessary to become an experienced neuroendocrine neurosurgeon as the authors state. On the contrary, I presume that some colleagues might even have more problems in understanding the regional anatomy when they always rely on neuronavigation. Thus, in my opinion, neuronavigation can obviously be used to verify both the midline (since the osseous skull base structures are not a subject of any movement during surgery) and the actual depth of the approach during surgery, and therefore might be helpful to avoid X-ray delivery to both the surgeon and the patient. However, in my experience, the anatomical key landmarks such as the vomer should be recognized in any case before the surgeon relies on a navigational data set. I believe that the technology that is described by the authors is of particular use in recurrent adenoma surgery, when it might be very difficult to dissect and visualize these residual bony landmarks.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yu, S., Wang, R. et al. Clinical application of a neuronavigation system in transsphenoidal surgery of pituitary macroadenoma. Neurosurg Rev 29, 306–312 (2006). https://doi.org/10.1007/s10143-006-0031-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-006-0031-y