Abstract
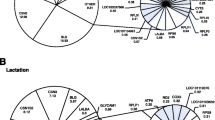
The mammary gland undergoes dramatic functional and metabolic changes during the transition from late pregnancy to lactation. To better understand the molecular events underlying these changes, we analyzed expression profiles of approximately 23,000 gene transcripts in bovine mammary tissue about day 5 before parturition and day 10 after parturition. At the cutoff criteria of the signed fold change ≥2 or ≤−2 and false discovery rate (FDR) ≤0.1, a total of 389 transcripts (1.6%) were significantly differentially expressed at the two stages. Of these transcripts with significant changes, 105 were up-regulated while 284 were down-regulated. Gene ontology analysis showed that the main up-regulated genes were those associated with transport activity (amino acid, glucose, and ion transporters), lipid and carbohydrate metabolism (lipoprotein lipase, acetyl-Coenzyme A synthetases, 6-phosphofructo-2-kinase, etc.), and cell signaling factors (protein p8, Rab18, etc.). The main down-regulated genes were associated with cell cycle and proliferation (cyclins, cell division cycle associated proteins, etc.), DNA replication and chromosome organization (centromere proteins, minichromosome maintenance proteins, histone, etc.), microtubule-based processes (microtubule associated protein tau, kinesin, tubulins, etc.), and protein and RNA degradation (proteasome, proteasome activator, RNA binding motif protein, etc.). The increased expression of glucose transporter GLUT1 mRNA during lactation was verified by quantitative reverse transcription/polymerase chain reactin (PCR) (P < 0.05). GLUT1 protein also increased twofold during lactation (P < 0.05). Furthermore, GLUT1 protein was primarily localized in mammary ductal epithelia and blood vessel endothelia before parturition, but was predominantly localized in the basolateral and apical membranes of mammary alveolar epithelial cells during lactation. Our microarray data provide insight into the molecular events in the mammary gland at the onset of lactation, indicating the up-regulation of genes involved in milk synthesis concomitant with the inhibition of those related to cell proliferation.




Similar content being viewed by others
References
Anderson RR, Harness JR, Snead AF, Salah MS (1981) Mammary growth pattern in goats during pregnancy and lactation. J Dairy Sci 64:427–432
Bell AW (1995) Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci 73:2804–2819
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125:279–284
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics (Oxf., England) 19:185–193
Davis AJ, Fleet IR, Goode JA, Hamon MH, Walker FM, Peaker M (1979) Changes in mammary function at the onset of lactation in the goat: correlation with hormonal changes. J Physiol 288:33–44
Farr VC, Stelwagen K, Cate LR, Molenaar AJ, McFadden TB, Davis SR (1996) An improved method for the routine biopsy of bovine mammary tissue. J Dairy Sci 79:543–549
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics (Oxf., England) 20:307–315
Hang J, Rillema JA (1997) Prolactin's effects on lipoprotein lipase (LPL) activity and on LPL mRNA levels in cultured mouse mammary gland explants. Proc Soc Exp Biol Med 214:161–166
Holt C (1983) Swelling of Golgi vesicles in mammary secretory cells and its relation to the yield and quantitative composition of milk. J Theor Biol 101:247–246
Hovey RC, Trott JF, Vonderhaar BK (2002) Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7:17–38
Hudson ER, Ma LS, Wilde CJ, Flint DJ, Baldwin SA (1997) Regulation of GLUT1 expression in the mammary gland. Biochem Soc Trans 25:464S
Icking A, Matt S, Opitz N, Wiesenthal A, Muller-Esterl W, Schilling K (2005) NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J Cell Sci 118:5059–5069
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31:e15
Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M (2006) From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34:D354–357
Lacasse P, Farr VC, Davis SR, Prosser CG (1996) Local secretion of nitric oxide and the control of mammary blood flow. J Dairy Sci 79:1369–1374
Lemkin PF, Thornwall GC, Walton KD, Hennighausen L (2000) The microarray explorer tool for data mining of cDNA microarrays: application for the mammary gland. Nucleic Acids Res 28:4452–445
Li RW, Meyer MJ, Van Tassell CP, Sonstegard TS, Connor EE, Van Amburgh ME, Boisclair YR, Capuco AV (2006) Identification of estrogen-responsive genes in the parenchyma and fat pad of the bovine mammary gland by microarray analysis. Physiol Genomics. 27:42–53
Liu X, Robinson GW, Hennighausen L (1996) Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol 10:1496–1506
Mellenberger RW, Bauman DE (1974) Metabolic adaptations during lactogenesis. Lactose synthesis in rabbit mammary tissue during pregnancy and lactation. Biochem J 142:659–665
Mellenberger RW, Bauman DE, Nelson DR (1973) Metabolic adaptations during lactogenesis. Fatty acid and lactose synthesis in cow mammary tissue. Biochem J 136:741–748
Neville MC, McFadden TB, Forsyth I (2002) Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66
Prosser CG, Davis SR, Farr VC, Lacasse P (1996) Regulation of blood flow in the mammary microvasculature. J Dairy Sci 79:1184–1197
Rijnkels M, Wheeler DA, de Boer HA, Pieper FR (1997) Structure and expression of the mouse casein gene locus. Mamm Genome 8:9–15
Robinson GW, McKnight RA, Smith GH, Hennighausen L (1995) Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development (Camb., England) 121:2079–2090
Rosen JM, Woo SL, Comstock JP (1975) Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry 14:2895–2903
Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC (2003) Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia 8:287–307
Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ, Stein T, Anderson SM, Neville MC (2007) Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28:323–336
Ruiz-Hidalgo MJ, Gubina E, Tull L, Baladron V, Laborda J (2002) dlk modulates mitogen-activated protein kinase signaling to allow or prevent differentiation. Exp Cell Res 274:178–188
Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Muller-Esterl W, Icking A (2006) Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell 17:3870–3880
Shuster RC, Houdebine LM, Gaye P (1976) Studies on the synthesis of casein messenger RNA during pregnancy in the rabbit. Eur J Biochem 71:193–199
Sorensen MT, Norgaard JV, Theil PK, Vestergaard M, Sejrsen K (2006) Cell turnover and activity in mammary tissue during lactation and the dry period in dairy cows. J Dairy Sci 89:4632–4639
Spychala J (2000) Tumor-promoting functions of adenosine. Pharmacol Ther 87:161–173
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Storey JD, Xiao W, Leek JT, Tompkins RG, Davis RW (2005) Significance analysis of time course microarray experiments. Proc Natl Acad Sci U S A 102:12837–12842
Suchyta SP, Sipkovsky S, Halgren RG, Kruska R, Elftman M, Weber-Nielsen M, Vandehaar MJ, Xiao L, Tempelman RJ, Coussens PM (2003) Bovine mammary gene expression profiling using a cDNA microarray enhanced for mammary-specific transcripts. Physiol Genomics 16:8–18
The Gene Ontology (GO) Project in 2006 (2006) Nucleic Acids Res 34:D322–326
Tucker HA (2000) Hormones, mammary growth, and lactation: a 41-year perspective. J Dairy Sci 83:874–884
Workman C, Jensen LJ, Jarmer H, Berka R, Gautier L, Nielser HB, Saxild HH, Nielsen C, Brunak S, Knudsen S (2002) A new non-linear normalization method for reducing variability in DNA microarray experiments. Genome Biol 3:research0048
Yang J, Kennelly JJ, Baracos VE (2000a) Physiological levels of Stat5 DNA binding activity and protein in bovine mammary gland. J Anim Sci 78:3126–3134
Yang J, Kennelly JJ, Baracos VE (2000b) The activity of transcription factor Stat5 responds to prolactin, growth hormone, and IGF-I in rat and bovine mammary explant culture. J Anim Sci. 78:3114–3125
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Zhao F-Q, Keating AF (2007) Expression and regulation of glucose transporters in bovine mammary gland (review). J Dairy Sci 90(Suppl 1):E76–E86
Zhao F-Q, Glimm DR, Kennelly JJ (1993) Distribution of mammalian facilitative glucose transporter messenger RNA in bovine tissues. Int J Biochem 25:1897–1903
Zhao F-Q, Dixon WT, Kennelly JJ (1996) Localization and gene expression of glucose transporters in bovine mammary gland. Comp Biochem Physio 115:127–134
Zhao F-Q, Miller PJ, Wall EH, Zheng Y-C, Dong B, Neville MC, McFadden TB (2004) Bovine glucose transporter GLUT8: cloning, expression, and developmental regulation in mammary gland. Biochim Biophys Acta 1680:103–113
Acknowledgements
We gratefully acknowledge the assistance of Pamela Bentley, Emma Wall, and Jeffrey White, Department of Animal Science, University of Vermont, in the mammary tissue biopsies. We also acknowledge the excellent technical support of Scott Tighe and Timothy Hunter, Vermont Cancer Center, in microarray analysis. This project was supported by National Research Initiative Competitive Grant no. 2007-35206-18037 from the USDA Cooperative State Research, Education, and Extension Service (to FQZ), the University of Vermont Agriculture Experiment Station (to FQZ) and Alberta Agriculture of Canada (to JJK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finucane, K.A., McFadden, T.B., Bond, J.P. et al. Onset of lactation in the bovine mammary gland: gene expression profiling indicates a strong inhibition of gene expression in cell proliferation. Funct Integr Genomics 8, 251–264 (2008). https://doi.org/10.1007/s10142-008-0074-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-008-0074-y