Abstract
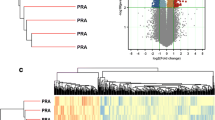
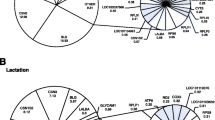
Many attempts have been made to identify estrogen-responsive genes using high-throughput approaches such as microarray, serial analysis of gene expression (SAGE), and in silico prediction. However, few studies have systematically analyzed regulatory networks and pathways affected by estrogen. In this report, we analyzed transcript profiles obtained from 16 prepubertal heifers in a 2 × 2 factorial experiment, with ovarian status (intact or ovariectomized) as the first factor and estrogen treatment as the second (control or estradiol). After 54 h of estrogen treatment, gene expression was evaluated in the parenchyma and fat pad of the bovine mammary gland using a high-density oligonucleotide microarray. The genes significantly regulated by estrogen were subject to pathway and regulatory network analysis using Ingenuity Pathways Analysis software. Approximately 2,344 genes responded significantly to estrogen treatment. Of these, 1016 genes were influenced by estrogen regardless of tissue or ovarian status, while the remaining genes were significant in one of four specific effects of tissue or ovarian status. The canonical pathways significantly regulated by estrogen (P < 0.05) included protein ubiquitination, G2/M cell cycle control, IGF1 signaling, N-glycan biosynthesis, sterol biosynthesis, and oxidative phosphorylation. A total of 23 regulatory networks were identified as estrogen responsive. The results provide insight into the molecular mechanisms through which estrogen regulates bovine mammary gland growth and development, supporting the concept that interaction between tissues within the mammary gland promotes mammary epithelial growth.





Similar content being viewed by others
References
Alarid ET, Bakopoulos N, Solodin N (1999) Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol 13:1522–1534
Apweiler R, Hermjakob H, Sharon N (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta 1473:4–8
Berthois Y, Dong XF, Roux-Dossetto M, Martin PM (1990) Expression of estrogen receptor and its messenger ribonucleic acid in the MCF-7 cell line: multiparametric analysis of its processing and regulation by estrogen. Mol Cell Endocrinol 74:11–20
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Carson DD, Farrar JD, Laidlaw J, Wright DA (1990) Selective activation of the N-glycosylation apparatus in uteri by estrogen. J Biol Chem 265:2947–2955
Castoria G, Migliaccio A, Bilancio A, Di DM, de FA, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F (2001) PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J 20:6050–6059
Chang EC, Frasor J, Komm B, Katzenellenbogen BS (2006) Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 147:4831–4842
Charpentier AH, Bednarek AK, Daniel RL, Hawkins KA, Laflin KJ, Gaddis S, MacLeod MC, Aldaz CM (2000) Effects of estrogen on global gene expression: identification of novel targets of estrogen action. Cancer Res 60:5977–5983
Chen ZJ, Parent L, Maniatis T (1996) Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell 84:853–862
Conaway RC, Brower CS, Conaway JW (2002) Emerging roles of ubiquitin in transcription regulation. Science 296:1254–1258
Connor EE, Wood DL, Sonstegard TS, da Mota AF, Bennett GL, Williams JL, Capuco AV (2005) Chromosomal mapping and quantitative analysis of estrogen-related receptor alpha-1, estrogen receptors alpha and beta and progesterone receptor in the bovine mammary gland. J Endocrinol 185:593–603
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Felty Q, Roy D (2005) Estrogen, mitochondria, and growth of cancer and non-cancer cells. J Carcinog 4:1
Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574
Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S (2000) Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res 60:321–327
Hovey RC, Trott JF, Vonderhaar BK (2002) Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7:17–38
Jin VX, Leu YW, Liyanarachchi S, Sun H, Fan M, Nephew KP, Huang TH, Davuluri RV (2004) Identifying estrogen receptor alpha target genes using integrated computational genomics and chromatin immunoprecipitation microarray. Nucleic Acids Res 32:6627–6635
Kassem M, Okazaki R, De LD, Harris SA, Robinson JA, Spelsberg TC, Conover CA, Riggs BL (1996) Potential mechanism of estrogen-mediated decrease in bone formation: estrogen increases production of inhibitory insulin-like growth factor-binding protein-4. Proc Assoc Am Physicians 108:155–164
Kinyamu HK, Chen J, Archer TK (2005) Linking the ubiquitin-proteasome pathway to chromatin remodeling/modification by nuclear receptors. J Mol Endocrinol 34:281–297
Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM (1999) Regulation of striatal dopamine receptors by estrogen. Synapse 34:222–227
Lesnefsky EJ, Hoppel CL (2006) Oxidative phosphorylation and aging. Ageing Res Rev 5:402–433
Li RW, Li C (2006) Butyrate induces profound changes in gene expression related to multiple signal pathways in bovine kidney epithelial cells. BMC Genomics 7:234
Li L, Li Z, Sacks DB (2005) The transcriptional activity of estrogen receptor-alpha is dependent on Ca2+/calmodulin. J Biol Chem 280:13097–13104
Li RW, Meyer MJ, Van Tassell CP, Sonstegard TS, Connor EE, Van Amburgh ME, Boisclair YR, Capuco AV (2006) Identification of estrogen-responsive genes in the parenchyma and fat pad of the bovine mammary gland by microarray analysis. Physiol Genomics 27:42–53
Meyer MJ, Capuco AV, Boisclair YR, Van Amburgh ME (2006) Estrogen-dependent responses of the mammary fat pad in prepubertal dairy heifers. J Endocrinol 190:819–827
Musters S, Coughlan K, McFadden T, Maple R, Mulvey T, Plaut K (2004) Exogenous TGF-beta1 promotes stromal development in the heifer mammary gland. J Dairy Sci 87:896–904
Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW (1999) Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862
Olden K, Bernard BA, White SL, Parent JB (1982) Function of the carbohydrate moieties of glycoproteins. J Cell Biochem 18:313–335
Qin X, Byun D, Strong DD, Baylink DJ, Mohan S (1999) Studies on the role of human insulin-like growth factor-II (IGF-II)-dependent IGF binding protein (hIGFBP)-4 protease in human osteoblasts using protease-resistant IGFBP-4 analogs. J Bone Miner Res 14:2079–2088
Ruan W, Newman CB, Kleinberg DL (1992) Intact and amino-terminally shortened forms of insulin-like growth factor I induce mammary gland differentiation and development. Proc Natl Acad Sci USA 89:10872–10876
Saito T, Tanaka R, Wataba K, Kudo R, Yamasaki H (2004) Overexpression of estrogen receptor-alpha gene suppresses gap junctional intercellular communication in endometrial carcinoma cells. Oncogene 23:1109–1116
Sherr CJ, Roberts JM (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18:2699–2711
Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541
Smith LJ, Henderson JA, Abell CW, Bethea CL (2004) Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 29:2035–2045
Soulez M, Parker MG (2001) Identification of novel oestrogen receptor target genes in human ZR75-1 breast cancer cells by expression profiling. J Mol Endocrinol 27:259–274
Stirone C, Duckles SP, Krause DN, Procaccio V (2005) Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68:959–965
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Tang S, Tan SL, Ramadoss SK, Kumar AP, Tang MH, Bajic VB (2004) Computational method for discovery of estrogen responsive genes. Nucleic Acids Res 32:6212–6217
Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T (1994) Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269:16433–16442
Vijay IK (1998) Developmental and hormonal regulation of protein N-glycosylation in the mammary gland. J Mammary Gland Biol Neoplasia 3:325–336
Wang DY, Fulthorpe R, Liss SN, Edwards EA (2004) Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene: EEIG1. Mol Endocrinol 18:402–411
Zhang Z, Chen K, Shih JC, Teng CT (2006) Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol Endocrinol 20:1547–1561
Acknowledgments
The authors thank Joy Castano for her excellent technical assistance. We acknowledge the contributions of our coauthors, Matthew J. Meyer, Curtis. P. Van Tassell, Tad S. Sonstegard, Erin E. Connor, Michael E. Van Amburgh, and Yves R. Boisclair, on the original study upon which this report is based. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
This table presents the 2,344 genes significantly regulated by estrogen in bovine mammary glands at FDR < 10%. (XLS 577 kb)
Supplementary File 2
This file contains all 23 regulatory networks influenced by estrogen with Ingenuity Pathways Analysis scores >7. (DOC 2930 kb)
Rights and permissions
About this article
Cite this article
Li, R.W., Capuco, A.V. Canonical pathways and networks regulated by estrogen in the bovine mammary gland. Funct Integr Genomics 8, 55–68 (2008). https://doi.org/10.1007/s10142-007-0055-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-007-0055-6