Abstract
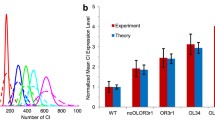
The biology and behavior of bacteriophage λ regulation have been the focus of classical investigations of molecular control of gene expression. Both qualitative and quantitative aspects of this behavior have been systematically characterized experimentally. Complete understanding of the robustness and stability of the genetic circuitry for the lysis-lysogeny switch remains an unsolved puzzle. It is an excellent test case for our understanding of biological behavior of an integrated network based on its physical, chemical, DNA, protein, and functional properties. We have used a new approach to non-linear dynamics to formulate a new mathematical model, performed a theoretical study on the phage λ life cycle, and solved the crucial part of this puzzle. We find a good quantitative agreement between the theoretical calculation and published experimental observations in the protein number levels, the lysis frequency in the lysogen culture, and the lysogenization frequency for mutants of OR. We also predict the desired robustness for the λ genetic switch. We believe that this is the first successful example in the quantitative calculation of robustness and stability of the phage λ regulatory network, one of the simplest and most well-studied regulatory systems.



Similar content being viewed by others
References
Ackers GK, Johnson AD, Shea MA (1982) Quantitative model for gene regulation by λ phage repressor. Proc Natl Acad Sci USA 79:1129–1133
Ao P (2002) Stochastic force defined evolution in dynamical systems. Submitted to Phys Rev Lett (physics/0302081)
Arkin A, Ross J, McAdams HH (1998) Stochastic kinetic analysis of developmental pathway bifurcation in phage lambda-infected Escherichia coli cells. Genetics 149:1633–1648
Aurell E, Sneppen K (2002) Epigenetics as a first exit problem. Phys Rev Lett 88:048101–1-4
Aurell E, Brown S, Johanson J, Sneppen K (2002) Stability puzzle in phage, λ. Phys Rev E 65:051914–1-9
Calef E, Avitabile LdG, Marchelli C, Menna T, Neubauer Z, Soller A (1971) The genetics of the anti-immune phenotype of defective lambda glycogens. In: Hershey AD (ed) The bacteriophage lambda. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 609–620
Darling PJ, Holt JM, Ackers GK (2000) Coupled energetics of λ cro repressor self-assembly and site-specific DNA operator binding. II. Cooperative interactions of Cro dimers. J Mol Biol 302:625–638
Dodd IB, Perkins AJ, Tsemitsidis DT, Egan JB (2001). Octamerization of λ CI repressor is needed for effective repression of PRM and efficient switching from lysogeny. Genes Dev 15:3013–3022
Eisen H, Brachet P, Pereira da Silva L, Jacob F (1970) Regulation of repressor expression in λ. Proc Natl Acad Sci USA 66:855–862
Hochschild A, Douhan J III, Ptashne M (1986) How λ repressor and λ Cro distinguish between OR1 and OR3. Cell 47:807–816
Jana R, Hazbun TR, Mollah AKMM, Mossing MC (1997) A folded monomeric intermediate in the formation of lambda Cro dimer-DNA complexes. J Mol Biol 273:402–416
Kim JG, Takeda Y, Matthews BW, Anderson WF (1987) Kinetic studies on Cro repressor-operator DNA interaction. J Mol Biol 196:149–158
Koblan KS, Ackers GK (1991) Energetics of subunit dimerization in bacteriophage lambda cI repressor: linkage to protons, temperature and KCl. Biochemistry 30:7817–7821
Koblan KS, Ackers GK (1992) Site-specific enthalpic regulation of DNA transcription at bacteriophage λ OR. Biochemistry 31:57–65
Kramers HA (1940) Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7:284
Kwon C, Ao P, Thouless DJ (2003) Structure of stochastic dynamics near fixed points. Proc Natl Acad Sci USA (in press)
Little JW, Shepley DP, Wert DW (1999) Robustness of a gene regulatory circuit. EMBO J 18:4299–4307
Pakula AA, Young VB, Sauer RT (1986) Bacteriophage λ cro mutations: effects on activity and intracellular degradation. Proc Natl Acad Sci USA 83:8829–8833
Ptashne M (1992) A genetic switch: phage λ and higher organisms, 2nd edition, Cell Press & Blackwell Scientific, Oxford
Ptashne M, Gann A (2002) Genes and signals. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Reik W, Dean W (2002) Back to the beginning: epigenetic reprogramming. Nature 420:127
Reinitz J, Vaisnys JR (1990) Theoretical and experimental analysis of the phage lambda genetic switch implies missing levels of cooperativity. J Theor Biol 145:295–318
Rozanov DV, D’Ari R, Sineoky SP (1998) RecA-independent pathways of lambdoid prophage induction in Escherichia coli. J Bacteriol 180:6306–6315
Sagai M, Wolynes PG (2003) Stochastic gene expression as a many-body problem. Proc Natl Acad Sci USA 100:2374–2379
Shea MA, Ackers GK (1985) The OR control system of bacteriophage lambda, a physical-chemical model for gene regulation. J Mol Biol 181:211–230
Takeda Y, Sarai A, Rivera VM (1989) Analysis of the sequence-specific interactions between Cro repressor and operator DNA by systematic base substitution experiment. Proc Natl Acad Sci USA 86:439–443
Takeda Y, Ross PD, Mudd CP (1992) Thermodynamics of Cro protein-DNA interactions. Proc Natl Acad Sci USA 89:8180–8184
Acknowledgements
We thank G.K. Ackers, D. Galas, and J.W. Little for valuable comments and critical discussions. This work was supported in part by the Institute for Systems Biology (P.A. and L.H.) by USA NIH grant under HG002894-01 (P.A.) and by a USA NSF grant under DMR 0201948 (L.Y.).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1. Details for Eq. 1
Following Ackers et al. (1982)and Aurell et al. (2002), we encode a state s of CI and/or Cro bound to OR by three numbers (i, j, k) referring to OR3, OR2 and OR1, respectively. The coding is 0 if the corresponding site is free, 1 if the site is occupied by a CI dimer, 2 if the site is occupied by a Cro dimer, and 3 if the site is occupied by an RNAp. The probability of a state s with i s CI dimers and js Cro dimers bound to OR is in the grand canonical approach of Shea and Ackers (1985)
For example, if CI occupies OR1, Cro OR2 and OR3, we have is =1, js =2, ks =0, and PR(s) = PR(221). There are a total of 40 states represented by s. The normalization constant Z is determined by summing over s: Z = Σs[CI]is[Cro]js[RNAp]ks exp(-G(s)/T).
The converting factor between protein number and concentration inside the bacterium is estimated to be 1.5×10-11.
RNAp occupies either OR1 and OR2, or OR2 and OR3, not other configurations. We further simplify the expression of PR(s) by noticing that the controlling of OR is operated by CI and Cro proteins, not RNAp (Ptashne 1992). If OR1 and OR2 is unoccupied by either CI or Cro, RNAp binds to them with a probability determined by RNAp binding energy. The case that RNAp first binds to OR1 and OR2, then blocking the CI and Cro binding is excluded based on the assumption that only CI and Cro controls the regulatory behavior. In addition to experimental observation, this assumption is justifiable if the time scale associated with CI and Cro binding is shorter than the RNAp binding. Except for an overall constant, which we include into the rate of transcription, the RNAp binding is no longer relevant. We therefore take it out of the expression PR(s) and drop the R subscript. The total number of states is reduced to 27. This simplification was first used by Aurell and Sneppen (2002).
The dimer and monomer numbers are determined by the formation and de-association of dimers, which gives the relation of dimer concentration to the total concentration of proteins as:
Here ΔGCI =−11.1 kcal/mol is the dimer association free energy for CI.
Similar expression for [Cro] is:
Here ΔGCro =−7 kcal/mol is the dimer association free energy for Cro. CI and Cro are produced from mRNA transcripts of cI and cro, which are initiated from promoter sites PRM and PR. The rate of initiation of transcription from PRM when stimulated by CI bound to OR2 is denoted TRM, and when not stimulated TRM u. The number of CI molecules produced per transcript is EcI. The overall expected rate of CI production is:
Similarly, the overall expected rate of Cro production is:
Putting together the production and decay of proteins, we obtain coupled equations for the time rate of change of numbers of CI and Cro in a cell (Reinitz and Vaisnys 1990)
The need to include fluctuations has been emphatically discussed in recent publications (Arkin et al. 1998; Aurell et al. 2002).
2. Parameters
We use basically the same set of parameters as listed in Table 1 as that of Aurell and Sneppen (2002). Those values were taken from various sources (Koblan and Ackers 1991, 1992; Takeda et al. 1989, 1992; Kim et al. 1987; Jana et al. 1997; Darling et al. 2000).
- RT:
-
= 0.617 kcal/mol,
- Effective bacterial volume:
-
= 0.7×10-15 l,
- Ecro :
-
= 20,
- EcI :
-
= 1,
- TRM :
-
= 0.115/s,
- TRM u :
-
= 0.01045/s,
- TR :
-
= 0.30/s,
- τCI :
-
= 2,943 s,
- τCro :
-
= 5,194 s
In vitro free energy differences:
- ΔG (001):
-
= −12.5 kcal/mol,
- ΔG (010):
-
= −10.5 kcal/mol,
- ΔG (100):
-
= −9.5 kcal/mol,
- ΔG (011):
-
= −25.7 kcal/mol,
- ΔG (110):
-
= −22.0 kcal/mol,
- ΔG (111):
-
= −35.4 kcal/mol,
- ΔG (002):
-
= −14.4 kcal/mol,
- ΔG (020):
-
= −13.1 kcal/mol,
- ΔG (200):
-
= −15.5 kcal/mol.
In vitro free energy differences for Hochschild mutant:
- ΔG (100):
-
= −10.5 kcal/mol,
- ΔG (200):
-
= −13.7 kcal/mol.
In vivo free energy differences = in vitro free energy differences + ΔG. This ΔG is –2.5 kcal/mol for CI, −4.0 kcal/mol for Cro, as found in our numerical calculations. These two variations between in vivo and in vitro free energy difference are less than 30%. The ΔG for cooperation energy is –3.7 kcal/mol, comparing with the in vitro value of –2.7 kcal/mol. This relatively large difference may be due to the looping effect (Dodd et al. 2001) implicitly considered in our present model.
3. Decomposition leading from Eq. 1 to Eq. 2
Because the mathematical method we use to analyze the stochastic equation Eq. 1 is new, we will describe briefly its essence. The method provides a visualization of the network dynamics. Its equivalence has been widely used in physics and chemistry. We start by rewriting Eq. 1 so that its notation is more familiar. Defining a vector r = (x, y), x=NCI, y=NCro, Eq. 1 becomes
with Fx = fCI(r) − x/τCI, Fy = fCro(r) − y/τCro, ζ x = ζ CI(r,t) and ζ y = ζ Cro(r,t). Equation 10 is supplemented by the relations
which define a diagonal diffusion matrix D. Equation 10 corresponds to the dynamics of a particle moving in two dimensional space with both deterministic and random forces. It is easy to check that ∇·F(r) ≠ 0 and ∇×F(r) ≠ 0 in general. Therefore F(r) cannot be represented by the gradient of a scalar potential. Recall that the simplest case in two dimensional motion when ∇·F(r) ≠ 0 and ∇×F(r) ≠ 0 is a charged particle moving in a magnetic field, Eq. 10 may be equivalent to
with B(r) the magnetic field, η the degradation matrix in the present paper (corresponding to the friction matrix), and the random or stochastic force ξ(r,t) which is related to the diagonal degradation matrix η,
Equation 12 is the same as Eq. 2 in the main text when S i,j = η i,j, A i,j = B εi,j, here εi,j= −εj,i, i, j = x, y.
It is easy to see that Eq. 10 can be derived from Eq. 12. However, deriving Eq. 12 from Eq. 10 is highly non-trivial. The procedure is described in Ao (2002) and Kwon et al. (2003). We briefly summarize their conclusions here:
4. Intermediate results and parameters
In Table 1, we have presented the final results. There are some intermediate results together with the parameters of interest.
The degradation constants: η11=0.056× τCI/NCI(lysogen), η22=0.056× τCro/NCro(lysis), here NCI(lysogen) is the NCI value at the lysogen point and NCro(lysis) the NCro value at the lysis point :
here 20% for λO R 121 and so on are the relative CI(Cro) level in lysogen (lysis).
We assume the degradation matrix is a diagonal constant matrix. Similar to Aurell and Sneppen (2002), we assume the fluctuations in Eq. 1 scale with the square root of protein number divided by relaxation time. This leads to the relation that degradation scales inversely with the protein number and proportionally to relaxation time, according to the Einstein relation (Ao 2002). The overall constant 0.056 is a fit to the experiment.
Barrier height from the lysogen minima to the saddle points:
Attempt frequency:
The lysogenization frequency is estimated assuming that at the early state of phage λ infection, before the decision mechanism is fully turned on, each phage acquires certain number of CI and Cro proteins. The CI and Cro protein numbers for different phage maybe different. The distribution of CI and Cro numbers for an ensemble of phage is assumed to be uniform. The distribution for the wild type is the inside area of a circle centered at NCI =50, NCro =0,
For different mutants growing under the same condition, we assume that the perimeter of such a distribution scale with the CI level in lysogen and the Cro level in lysis. For example, for λO R 121, the ellipse of the distribution is given by:
Here 20% and 100% are the relative CI level in lysogen and relative Cro level in lysis compared with the wildtype.
5. Communication from J.W. Little
J.W. Little, in private communication, pointed out a situation different from the assumption in the present paper that we treat all probability events, extrinsic and intrinsic, on an equal footing; our assumption that all are represented by the Gaussian white noise may be an oversimplified one. For example, some probability events, such as the prm240 mutation which may be equivalent to destroy the gene switch, as suggested by Little’s unpublished data, could have totally different biological consequences and therefore may not be well approximated by the Gaussian white noise in the present model. Neglecting such large, rare, and destructive events indeed enhances the gene switch stability against small and frequent Gaussian white noise. Particularly it may greatly reduce the wildtype switching rate. We found that in such cases other molecular parameters, such as the binding free energies, are nevertheless less sensitive to those rare events. The same overall good agreement with experimental data can be obtained. We will discuss this feature elsewhere in detail.
Rights and permissions
About this article
Cite this article
Zhu, XM., Yin, L., Hood, L. et al. Calculating biological behaviors of epigenetic states in the phage λ life cycle. Funct Integr Genomics 4, 188–195 (2004). https://doi.org/10.1007/s10142-003-0095-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-003-0095-5