Abstract
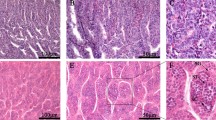
DNA methylation reprograms during gametogenesis and embryo development, which is essential for germ cell specification and genomic imprinting in mammals. Corresponding process remains poorly investigated in molluscs. Here, we examined global DNA methylation level in the gonads of scallop Patinopecten yessoensis during gametogenesis and in embryos/larvae at different stages. DNA methylation level fluctuates during gametogenesis and early development, peaking at proliferative stage of ovary, growing stage of testis, and in blastulae. To understand the mechanisms underlying these changes, we conducted genome-wide characterization of DNMT family and investigated their expression profiles based on transcriptomes and in situ hybridization. Three genes were identified, namely PyDNMT1, PyDNMT2, and PyDNMT3. Expression of PyDnmt3 agrees with DNA methylation level during oogenesis and early development, suggesting PyDNMT3 may participate in de novo DNA methylation that occurs mainly at proliferative stage of ovary and testis, and in blastulae and gastrulae. PyDnmt1 expression is positively correlated with DNA methylation level during spermatogenesis, and is higher at maturation stage of ovary and in 2–8 cell embryos than other stages, implying possible involvement of PyDNMT1 in DNA methylation maintenance during meiosis and embryonic development. This study will facilitate better understanding of the developmental epigenetic reprogramming in bivalve molluscs.






Similar content being viewed by others
References
Adrian B (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Allegrucci C, Thurston A, Lucas E, Young L (2005) Epigenetics and the germline. Reproduction 129:137–149
Branco MR, Masaaki O, Wolf R (2008) Safeguarding parental identity: Dnmt1 maintains imprints during epigenetic reprogramming in early embryogenesis. Genes Dev 22:1567–1571
Chen T, Yoshihide U, Jonathan ED, Wang Z, Li E (2003) Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol 23:5594–5605
Chin BA (2018) Characterizing the role of DNA methylation patterns in the California mussel, Mytilus californianus. (Masters dissertation, Sonoma State University)
Dean W, Ferguson SA (2001) Genomic imprinting: mother maintains methylation marks. Curr Biol 11:R527–R530
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Ewa B, K Naga M, Leonardo DA, M Cecilia C, J Richard C (2009) Identification of a region of the DNMT1 methyltransferase that regulates the maintenance of genomic imprints. Proc Natl Acad Sci U S A 106:20806–20811
Feng L, Li X, Yu Q, Ning X, Dou J, Zou J, Zhang L, Wang S, Hu X, Bao Z (2014) A scallop IGF binding protein gene: molecular characterization and association of variants with growth traits. PLoS One 9:e89039
Fu X et al (2013) Sequencing-based gene network analysis provides a core set of gene resource for understanding thermal adaptation in Zhikong scallop Chlamys farreri. Mol Ecol Resour 14:184–198
Gavery MR, Roberts SB (2013) Predominant intragenic methylation is associated with gene expression characteristics in a bivalve mollusc. Peerj 1:e215
Gavery MR, Roberts SB (2014) A context dependent role for DNA methylation in bivalves. Brief Funct Genomics 13:217–222
Goll MG et al (2006) Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 311:395–398
Hales BF, Lisanne G, Claudia L, Bernard R (2011) Epigenetic programming: from gametes to blastocyst birth defects research part a Clinical & Molecular. Teratology 91:652–665
Hisato K et al (2012) Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet 8:e1002440
Howell CY, Bestor TH, Ding F, Latham KE, Mertineit C, Trasler JM, Chaillet JR (2001) Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell 104:829–838
Hu X, Guo H, He Y, Wang S, Zhang L, Wang S, Huang X, Roy SW, Lu W, Hu J, Bao Z (2010) Molecular characterization of Myostatin gene from Zhikong scallop Chlamys farreri (Jones et Preston 1904). Genes Genet Syst 85:207–218
Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A (1992) Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev 6:705–714
Kenichiro H, Masaki O, Hong L, En L (2002) Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 129:1983–1993
Kumar S, Cheng X, Klimasauskas S, Mi S, Posfai J, Roberts RJ, Wilson GG (1994) The DNA (cytosine-5) methyltransferases. Nucleic Acids Res 22(1):1–10
Lan J et al (2013) Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell 153:773–784
LePage DP, Jernigan KK, Bordenstein SR (2014) The relative importance of DNA methylation and Dnmt2-mediated epigenetic regulation on Wolbachia densities and cytoplasmic incompatibility. PeerJ 2:e678
Li R (2018) Molecular basis of sex differentiation in Patinopecten yessoensis. (Doctoral dissertation, Ocean University of China)
Li R, Zhang R, Zhang L, Zou J, Xing Q, Dou H, Hu X, Zhang L, Wang R, Bao Z (2015) Characterizations and expression analyses of NF-κB and Rel genes in the Yesso scallop (Patinopecten yessoensis) suggest specific response patterns against gram-negative infection in bivalves. Fish Shellfish Immunol 44:611–621
Li Y et al (2016) Transcriptome sequencing and comparative analysis of ovary and testis identifies potential key sex-related genes and pathways in scallop Patinopecten yessoensis. Mar Biotechnol 18:1–13
Li Y, Sun X, Hu X, Xun X, Zhang J, Guo X, Jiao W, Zhang L, Liu W, Wang J, Li J, Sun Y, Miao Y, Zhang X, Cheng T, Xu G, Fu X, Wang Y, Yu X, Huang X, Lu W, Lv J, Mu C, Wang D, Li X, Xia Y, Li Y, Yang Z, Wang F, Zhang L, Xing Q, Dou H, Ning X, Dou J, Li Y, Kong D, Liu Y, Jiang Z, Li R, Wang S, Bao Z (2017) Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat Commun 8:1721
Li R et al (2018) FOXL2 and DMRT1L are Yin and Yang genes for determining timing of sex differentiation in the bivalve mollusk Patinopecten yessoensis. Front Physiol 9:1166
Malone T, Blumenthal RM, Cheng X (1995) Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyl-transferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol 253 (4):618–632
Marques CJ, João PM, Carvalho F, Bièche I, Barros A, Sousa M (2011) DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 6:1354–1361
Mendonça AS, Braga TF, Melo EO, Dode MAN, Franco MM (2018) Distribution of 5-methylcytosine and 5-hydroxymethylcytosine in bovine fetal tissue of the placenta. Pesqui Vet Bras 38 (10):2012–2018
Oakes CC, Salle SL, Smiraglia DJ, Robaire B, Trasler JM (2007) Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Dev Biol 307:368–379
Okano M, Xie S, Li E (1998) Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res 26:2536–2540
Okano M, Bell DW, Haber DA, Li E (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247–257
Olson CE, Roberts SB (2014) Genome-wide profiling of DNA methylation and gene expression in Crassostrea gigas male gametes. Front Physiol 5:224–224
Reik W, Walter J (2001) Genomic imprinting: parental influence on the genome. Nat Rev Genet 2:21–32
Remnant EJ, Ashe A, Young PE, Buchmann G, Beekman M, Allsopp MH, Suter CM, Drewell RA, Oldroyd BP (2016) Parent-of-origin effects on genome-wide DNA methylation in the cape honey bee (Apis mellifera capensis) may be confounded by allele-specific methylation. BMC Genomics 17:226
Riviere, Guillaume, Goux, Didier (2013) DNA methylation is crucial for the early development in the oyster C.gigas. Mar Biotechnol 15:739–753
Riviere G, He Y, Tecchio S, Crowell E, Gras M, Sourdaine P, Guo X, Favrel P (2017) Dynamics of DNA methylomes underlie oyster development. PLoS Genet 13:e1006807
Santos F, Hendrich B, Reik W, Dean W (2002) Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 241:172–182
Schübeler D (2015) Function and information content of DNA methylation. Nature 517 (7534):321–326
Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W (2012) The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48:849–862
Simon A, Paul Theodor P, Wolfgang H (2015) HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169
Smallwood SA, Tomizawa SI, Krueger F, Ruf N, Carli N, Segonds-Pichon A, Sato S, Hata K, Andrews SR, Kelsey G (2011) Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet 43:811–814
Stefanie S, Peat JR, Hore TA, Fátima S, Wendy D, Wolf R (2013) Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc B 368:20110330
Stewart KR, Veselovska L, Kelsey G (2016) Establishment and functions of DNA methylation in the germline. Epigenomics 8:1399–1413
Sun Y, Hou R, Fu X, Sun C, Wang S, Wang C, Li N, Zhang L, Bao Z (2014) Genome-wide analysis of DNA methylation in five tissues of Zhikong scallop, Chlamys farreri. Plos One 9:e86232
Surani MA (2001) Reprogramming of genome function through epigenetic inheritance. Nature 414:122–128
Sylvain F, Robert K, Matteo P, Suhua F, Jacobsen SE, Robinson GE, Ryszard M (2012) DNA methylation dynamics, metabolic fluxes, gene splicing, and alternative phenotypes in honey bees. Proc Natl Acad Sci U S A 109:4968–4973
Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9 (6):465–476
Wang R, Wang Z. (2008) Science of marine shellfish culture. Qingdao: Ocean University of China Press;
Wang X et al (2014) Genome-wide and single-base resolution DNA methylomes of the Pacific oyster Crassostrea gigas provide insight into the evolution of invertebrate CpG methylation. BMC Genomics 15:1–12
Wang S, Zhang J, Jiao W, Li J, Xun X, Sun Y, Guo X, Huan P, Dong B, Zhang L, Hu X, Sun X, Wang J, Zhao C, Wang Y, Wang D, Huang X, Wang R, Lv J, Li Y, Zhang Z, Liu B, Lu W, Hui Y, Liang J, Zhou Z, Hou R, Li X, Liu Y, Li H, Ning X, Lin Y, Zhao L, Xing Q, Dou J, Li Y, Mao J, Guo H, Dou H, Li T, Mu C, Jiang W, Fu Q, Fu X, Miao Y, Liu J, Yu Q, Li R, Liao H, Li X, Kong Y, Jiang Z, Chourrout D, Li R, Bao Z (2017) Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat Ecol Evol 1:120
Wiley KL, Treadwell E, Manigaba K, Word B, Lyn-Cook BD (2013) Ethnic differences in DNA methyltransferases expression in patients with Systemic Lupus Erythematosus. J Clin Immunol 33:342–348
Wolf R (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447:425–432
Yu J, Zhang L, Li Y, Li R, Zhang M, Li W, Xie X, Wang S, Hu X, Bao Z (2017) Genome-wide identification and expression profiling of the SOX gene family in a bivalve mollusc Patinopecten yessoensis. Gene 627:530–537
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PWH, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Lošo T, du Y, Sun X, Zhang S, Liu B, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinberg CEW, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54
Funding
This work was supported by the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0302-1), National Natural Science Foundation of China (31871499 and 31572600), Major basic research projects of Shandong Natural Science Foundation (ZR2018ZA0748) and Fundamental Research Funds for the Central Universities (201762001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, L., Li, Y. et al. Dynamics of DNA Methylation and DNMT Expression During Gametogenesis and Early Development of Scallop Patinopecten yessoensis. Mar Biotechnol 21, 196–205 (2019). https://doi.org/10.1007/s10126-018-09871-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-09871-w